What Will Happen When Dissolution Of Solid In Liquid?
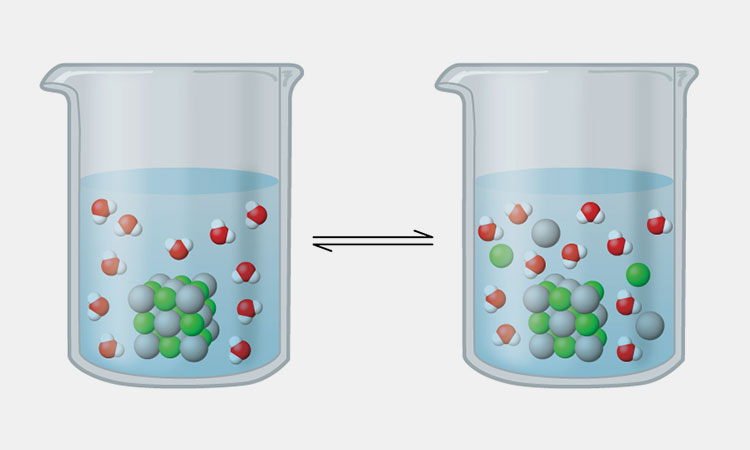
Solids Dissolve In Liquid-sourced: libretexts
The dissolution of solid in liquid is the process of forming a solution. Solutions are of great significance to the physiological activities of animals, plants and humans.
In agriculture, solutions can provide nutrients and moisture to plant cultivation and soil. In medical treatment, the solution can be made into glucose solution and physiological saline to prepare an injection solution for use. In the food industry, many drinks and prepared dishes require the preparation of solutions.
What will happen when dissolution of solid in liquid? How are the steps to dissolve the solid in liquid? Following this post, you may get the most complete information about dissolution of solid in liquid.
1.What Does It Mean The Dissolution Of Solid In Liquid?
What Does It Mean The Dissolution Of Solid In Liquid-sourced: thoughtco
When a solid and a liquid are completely mixed, the solid dissolves in the liquid, and it is called the dissolution of solid in liquid. The solid will break down into tiny pieces so that its particles are spread throughout the new mixture.
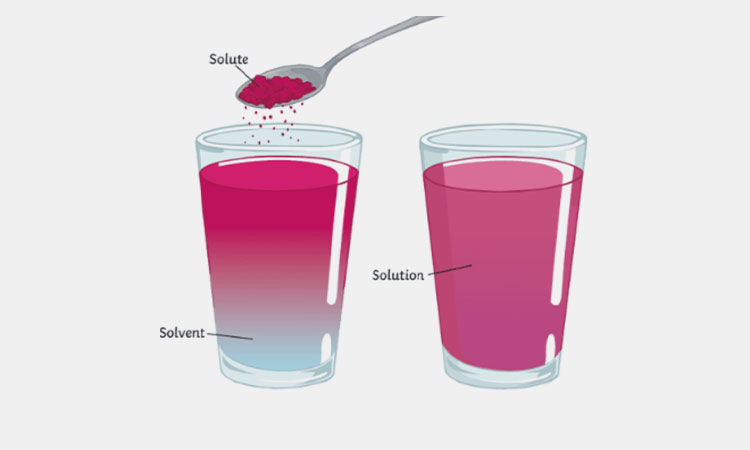
When a solid dissolves in a liquid, the dissolved substance is called a solute, the liquid in which they are dissolved is called a solvent, and the resulting mixture is called a solution.
Dissolution Of Solid In Liquid-sourced: facts
Water is a good solvent and absorbs impurities easily. Purified water is tasteless, colorless and odorless. It is often called a universal solvent. Dissolved solids are any minerals, salts, metals, cations or anions dissolved in water.
2.What Will Happen When Dissolution Of Solid In Liquid?
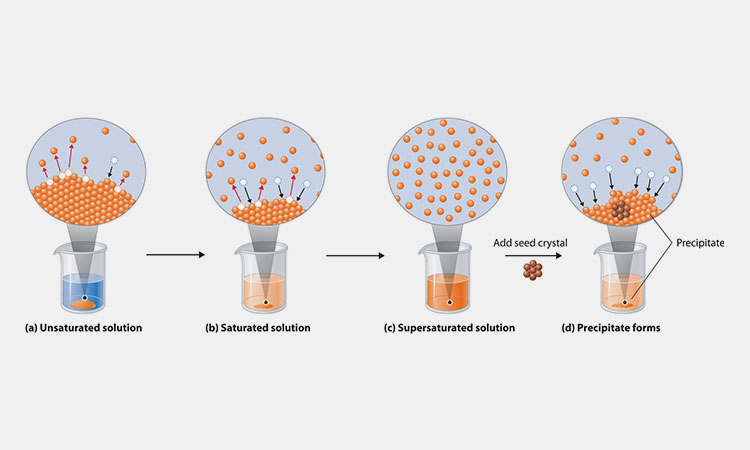
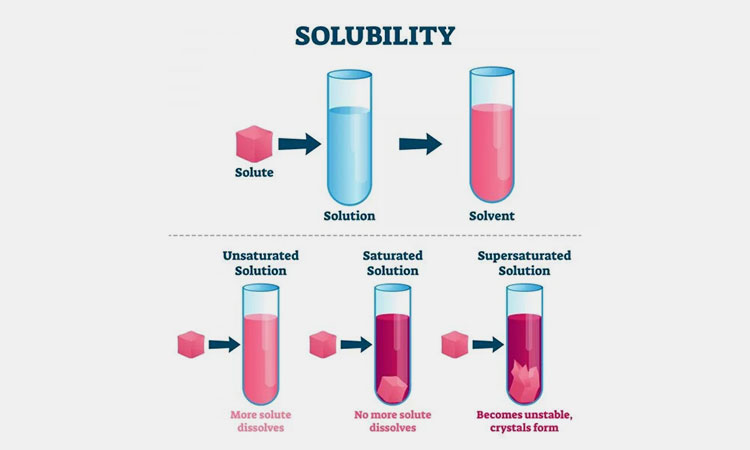
When a solid is sufficiently dissolved in a liquid, a mixture of solid and solution is produced. This mixture is homogeneous and stable. All parts of the solution have exactly the same concentration and properties.
When the external conditions do not change, or when the solvent does not evaporate and the temperature does not change, no solute will precipitate out of the solid in the solution.
Dissolution of a substance: The process by which a substance is dispersed uniformly into another substance in the form of molecules or ions.
There are 3 types of solutions:
Suspension
Suspension-sourced: solutionpharmacy
Substances are dispersed into water as fine solid particles, forming a heterogeneous and unstable mixture, such as muddy water (solid sedimentation).
Emulsion
Emulsion-sourced: rheonics
A substance is dispersed into water as small droplets, forming a non-uniform and unstable mixture, such as oil and water (liquid stratification).
Solution
Solution-sourced: thoughtco
A substance that dissolves to form a uniform, stable mixture.
What are the principles that solids dissolve in liquid? There are three basic principles when the solid dissolves in liquid:
Water and Alcohol-sourced: laballey
Some Solids Dissolve In Liquid Are Not Solutions
Uniform and stable liquids are not necessarily solutions. For example, water and alcohol are uniform and stable liquids, but they are not solutions, but pure substances.
Some Solids Dissolve In Liquid Are Not Colorless
The solution is not necessarily colorless. For example, potassium permanganate solution is purple, copper sulfate solution is blue, and ferric chloride solution is yellow.
Some Solids Dissolve In Liquid Are Not Liquid
A solution does not have to be a liquid. As long as it is a homogeneous and stable mixture, it is a solution, such as alloys, air, etc.
3.What Do You See When A Solid Dissolves In Liquid?
Dissolution occurs when solid particles dissolve in a liquid. As it dissolves, you'll see this solid particle kind of disappear. But it actually dissolves in the liquid and forms a clear solution.
The process of solids dissolve in liquid:
Process Of Solids Dissolve In Liquid-sourced: twinkl
Generally, solids are mixed into liquids to create a new liquid, called a solution. No heating is required during the entire dissolution process. When the solid particles are completely dissolved in the liquid, you no longer see the solid particles, but the entire liquid solution.
Notice:
Not all substances can be incorporated into liquids. Whether in actual operations or experiments, there will be solid substances that can never be dissolved in liquids.
4.What Are The Steps To Dissolve Solid In Liquid?
Steps To Dissolve Solid In Liquid-sourced: rsc
To dissolve the solid in liquid can be easy with the simple solids and liquids.
Step 1:
Take a few grains of table salt, sucrose, and potassium permanganate crystals and put them into three test tubes;
Step 2:
Add water and shake, and carefully observe the experimental phenomenon;
Step 3:
Prepare another 2 test tubes with a small amount of soil and cooking oil;
Step 4:
Add about 1/3 of the volume of water to the test tubes, shake, place, and observe the experimental phenomena;
Through different experiments, you can understand the phenomenon of substance dissolution. These laws include:
Color: When some substances are dissolved in water, the solution will show a certain color.
Temperature: The dissolution process may be endothermic or exothermic.
Through the substance dissolution phenomenon obtained after experiments, you can get the methods to quickly dissolve a solid substance:
Different Color Solution-sourced: newscientist
Stir constantly while dissolving;
Dissolve in hot water;
Grind into powder and dissolve;
5.What Facts Affect The Dissolution Of Solid In Liquid?
Facts Affect The Dissolution Of Solid In Liquid-sourced: techiescientist
Different factors also affect the dissolution of solid in liquid. The facts about the dissolution of solid in liquid include temperature, pressure, polarity and molecular size. It will increase or decrease the dissolution of solid in liquid.
Effect of Temperature On Solubility
Effect of Temperature on Solubility-sourced: saylordotorg
The solubility of most solids dissolved in water increases as temperature increases and decreases as temperature decreases. This is because higher temperatures increase the vibration, or kinetic energy, of solute molecules. Solute molecules are held together by intermolecular attraction.
The increase in kinetic energy weakens the intermolecular attraction, making it easier for the solvent molecules to break down the solute molecules, making them more readily soluble.
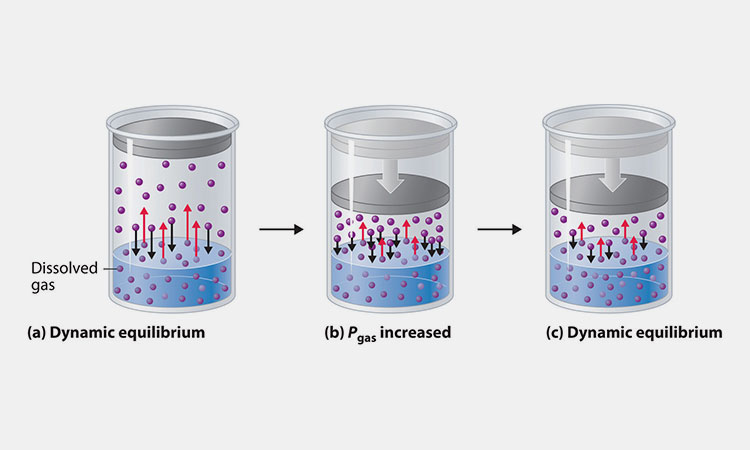
Effect of Pressure On Solubility
Effect of Pressure on Solubility-sourced: saylordotorg
The solubility of a gas in a solvent increases as the pressure increases. But the effect of pressure on the solubility of condensed phases (solid and liquid) is negligible.
Effect of Molecular Size On Solubility
Effect of Molecular Size on Solubility-sourced: saylordotorg
Solubility decreases with increasing molecular size. The larger the size of the molecules in the solute, the harder it is for the solvent molecules to wrap around them and dissolve them. On the other hand, solvent molecules more easily wrap around smaller sized molecules, thereby increasing the solubility of the substance.
Effect of Polarity On Solubility
Effect of Polarity on Solubility-sourced: gzscienceclassonline
In general, polar solutes are easier to dissolve in polar solvents, while non-polar solutes are easier to dissolve in non-polar solvents. Polar solutes will not dissolve in non-polar solvents and vice versa. The reason fats are insoluble in water is that fats are non-polar and water is polar.
6.What Are The Purposes To Dissolve Solids In Liquid?
To make solution by dissolving solids in liquid has a wide range of uses and meanings. Its application also has great effects and applications in agriculture, pharmaceutical industry and food:
In Agriculture Industry
In Agriculture Industry-sourced: mladenbalinovac
In agriculture, soil-less cultivation technology uses solutions instead of soil for cultivation to provide nutrients for plants. And in the process of cultivating plants, many pesticides and herbicides need to be sprayed out by preparing solutions by dissolving solids in liquid to achieve the purpose of killing insects and weeds.
In Pharmaceutical Industry
In Pharmaceutical Industry-sourced: nurse
In the pharmaceutical industry, many glucose solutions and physiological saline need to be formulated into solutions according to a certain proportion, so that syringes can be used to facilitate people's use. Also, in chemical experiments, many reactions are performed in solutions. Because this can speed up the rate of chemical reactions.
In Food Industry
In Food Industry-sourced: epicurious
Many foods require preservation in solutions. For example, specially treated brine can be used to store pickled cucumbers, etc. Canned fruits, etc., use special solutions to store and preserve processed fruits.
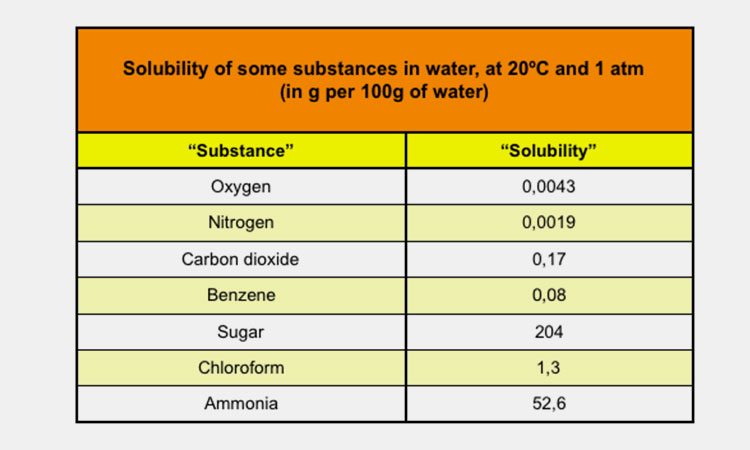
7.What Is The Solubility Of Solids In Liquid?
Solubility is the best indicator of a substance's ability to dissolve into a liquid.
Solubility:
Solubility-sourced: chemicals
Solubility is the physical property of one substance dissolving in another substance. Solubility depends on solute and solvent. Physical properties such as pressure and temperature also affect solubility.
Solubility Of Solids In Liquid:
Solubility Of Solids In Liquid-sourced: geeksforgeeks
When a solid solute is added to a liquid, the solute particles dissolve in the solvent, a process called dissolution. The solubility of solids depends on the interaction between solute and solvent. Strong solute and solvent attraction forces can lead to greater solubility. Weak solute-solvent attraction leads to smaller solubility.
Solubility is the relative ability of a solute to dissolve into a solvent. This ability can change due to many factors. Although pressure has a significant effect on gas solubility, temperature has a significant effect on liquid solubility.
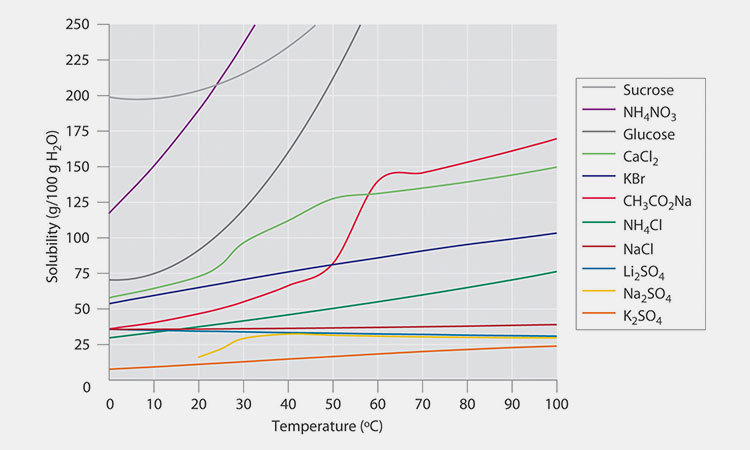
Different solid substances have different solubility at different temperatures:
Solubility of Different Solids-sourced:bioprofe
①As the temperature increases, the solubility increases;
②Solubility is not affected by temperature;
③Solubility decreases as temperature increases;
Conclusion:
Through this what will happen when dissolution of solid in liquid guide, you can learned that the dissolution of solid in liquid is both chemical reactions and physical changes. The wide application of dissolution of solid in liquid brings great improvement of the solution. Is there more you still want to know? Do not hesitate to contact us!
Don't forget to share this post!
Tablet Press Machine Related Posts
Tablet Press Machine Related Products
Tablet Press Machine Related Videos
CONTACT US
Tell us your raw material and project budget to get quotations within 24 hours.
WhatsApp Us: +86 181 6426 8586

Want the best price & newest pharmaceutical machinery buying guide,tips and trends sent straightly to your box?Sign up for AIPAK’s monthly newsletter,we’re free for your consultation and Offer you the most suitable solutions!
The Buyer's Guide
- Capsule Filling Buyer's Guide
- Blister Packaging Buyer's Guide
- Tablet Counting Buyer's Guide
- Tube Filling Buyer's Guide
- Cartoning Buyer's Guide
- Gummy Making Buyer's Guide
- CO2 Extraction Buyer's Guide
- Empty Capsules Buyer's Guide
- Suppository Filling Buyer's Guide
- Tablet Coating Buyer's Guide
- Tablet Press Buyer's Guide
- Softgel Encapsulation Buyer's Guide
Most Popular
- 7 Importance Of Pharmaceutical Packaging In Different Applications You Must Know
- 6 Advantages You Must Know About Tablet Counting Machine
- 8 Advantages of Blister Packaging You Must Know
- 6 Critical Applications of Automatic Capsule Filling Machine
- 6 Stations You must Know to Improve the Filling Quality of Automatic Capsule Filling Machine