What Are Pharmaceutical Packaging Materials? How To Choose It?
What are the pharmaceutical packaging materials? How to choose the best pharmaceutical packaging materials?
Packaging materials used for pharmaceutical tablets come into direct contact with the medicine and therefore belong to the category of pharmaceutical packaging.
Pharmaceuticals are a special commodity whose efficacy and quality are directly related to human health and safety, and the packaging materials and structure used must ensure the efficacy of the drug at the same time, but also play a role in ensuring the reliability and convenience of the use of drugs.
So as a pharmaceutical manufacturer, when selecting pharmaceutical packaging materials, it is necessary to understand some properties and characteristics of the packaging materials and containers, so as to reasonably and accurately select pharmaceutical packaging materials in combination with some special requirements of drugs.
Plastic bottle packaging
The most important feature of plastic bottles for pharmaceuticals is that they are light in quality, unbreakable, clean and beautiful, and can be used directly by pharmaceutical manufacturers without cleaning and drying.
And some of its technical indicators and a large number of data show that its chemical resistance, resistance to water vapour permeability sealing performance is excellent, it can fully play a safe shielding protection role for the drugs contained in the validity period.
The variety of materials used are high-density polyethylene bottles (HDPE), polypropylene bottles (PP), polycarbonate bottles (PC), polyester bottles (PET), the early 1990s,a new material for blow-moulded bottles diethyl naphthalene dicarboxylate (PEN) plastic bottles has been developed, its properties are better than PET, it is high strength, good heat resistance, UV radiation, carbon dioxide gas and Oxygen barrier performance, good chemical resistance, more extensive than PET, PEN material is a new type of polyester resin actively developed at home and abroad in recent years, is also an excellent material for pharmaceutical plastic bottle packaging.
How to choose the correct pharmaceutical packaging materials?
Due to the many specifications of pharmaceutical plastic bottles, small dozen millilitres, large thousands of millilitres, some colourless, some transparent, colourful, different shapes, a wide range of categories.
In the face of the exciting plastic bottle market, as a pharmaceutical manufacturer in the selection of plastic bottles mainly grasp the following principles:
Choose plastic bottle main raw materials, additives formula
Solid plastic bottles with product standards, respectively, the applicable main raw materials must meet the non-toxic, odorless and other requirements, as the main raw materials available for selection of a variety, which requires the comprehensive performance of raw materials to be selected, the general choice of tablets of high-density polyethylene, polypropylene bottles, such as the need for transparency can be used PET bottles, such as drugs need higher barrier performance, and light-blocking, opaque, the choice of brown PET bottle, better barrier performance for the PEN bottle.
Liquid dosage form drugs generally choose polypropylene bottles or polyester bottles as the main raw material.
Bottle and cover sealing, water vapour permeability
Sealing and water vapour permeability are two important technical indicators of pharmaceutical plastic bottles, which play an important role in the stability of the medicine.
Plastic bottle product quality standards
The quality of the product can be analyzed and judged from the quality standards of the production plant. Pharmaceutical plastic bottle enterprises should develop enterprise standards that are stricter than national standards and industry standards.
Quality assurance system
Audit of suppliers has become an essential and important part of the procurement of plastic bottles. Through the audit, the production plant's software and hardware facilities, technical equipment, quality comprehensive level to make a comprehensive and correct assessment.
Stability and compatibility of plastic bottles for drug filling
The selection of plastic bottles, especially new plastic bottles for new drugs (or new materials, new processes) should first be tested to examine the stability of the drug and the compatibility between the plastic bottle and the drug.
The permeation, dissolution, adsorption, chemical reaction and denaturation of drugs and plastic bottle materials must be determined by scientific testing.
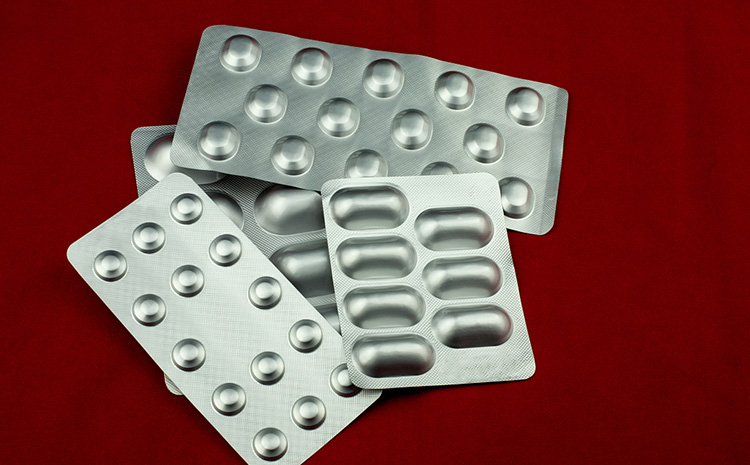
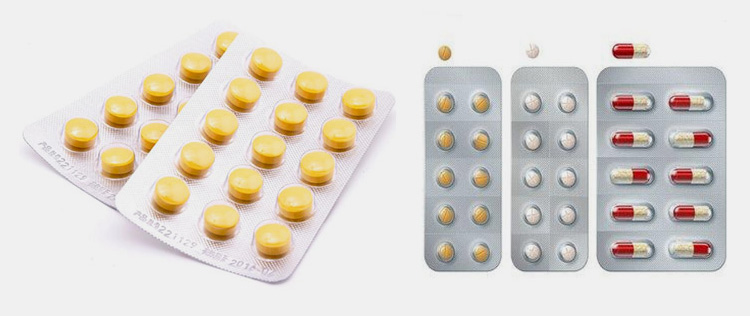
Blister packaging for pharmaceutical tablets
Blister packaging is also known as blister-eye packaging. Compared to bottled tablets, the biggest advantage of blister packaging is that it is easy to carry and can reduce contamination during the carrying and taking of medicines.
However, as a regular medication for patients, there is a single dose of larger, making the unit cost of blister packaging significantly higher than that of bottled drugs, some pharmaceutical companies, usually the price is higher, high technology and new varieties of drugs choose blister packaging most.
The materials used in blister packaging are mainly PTP pharmaceutical aluminium foil and plastic rigid sheets.
Pharmaceutical aluminium foil is a sealing material on plastic rigid sheets, which is based on rigid industrial pure aluminium and is non-toxic, corrosion resistant, impermeable, heat resistant, moisture resistant, light resistant and autoclavable.
Plastic rigid sheets are usually made of polyvinyl chloride (PVC), polyvinylidene chloride (PVDC) or composite materials. They have good barrier properties to water, steam and light.
Selection of pharmaceutical aluminium foil materials and principles
Before using PTP aluminium foil, the demander first signs a procurement contract with the supplier. In the contract on the technical requirements of the product must be written: product name, specifications (width and thickness requirements), printing colour, aluminium foil volume text arrangement direction and position; aluminium foil structure, adhesive coating amount, coating colour has no requirements, packaging requirements, etc.
Basic principles for the selection of pharmaceutical plastic rigid tablets or composite materials
For ordinary tablets, polyvinyl chloride (PVC) rigid tablets can be used if the requirements for moisture resistance are not high.
For drugs with moisture-proof and anti-oxidation requirements, or with longer shelf life requirements, PET hard tablets and PP hard tablets can be used. Composite hard tablets are available: PVC/PVDC or PVC/PE/PVDC. For oral liquids and suppositories, polyvinyl chloride and polyethylene laminates (PVC/PE) are available.
How do I choose a rigid tablet material for pharmaceutical blister packaging? General procedure.
According to the nature of the drug and shelf-life requirements to choose the use of what polymer rigid tablets, to be specific,you can consult the pharmaceutical packaging materials manufacturers technical experts, or material testing, to take full account of the versatility of equipment, the appearance of novelty, the lowest cost.
Conduct machine tests to determine the suitability of the pharmaceutical packaging material for the equipment and the satisfaction of the product quality and appearance.
Do stability and compatibility tests, which must be done for each drug because the nature of various drugs is different.
You can go to the drug packaging material manufacturers to carry out environmental, equipment, process, quality and other related management content audit; and together drug packaging material manufacturers to determine the drug packaging material raw materials, formulations and material structure and packaging material product standards, and finally signed an agreement.
Aluminium-plastic strip composite film packaging for pharmaceutical tablets
For some larger dosage forms of pharmaceutical tablets or UV sensitive, hygroscopic, require heat and cold resistance, and require a long validity period and improve the choice of packaging grade of pharmaceutical tablets, most of the strip composite film packaging, referred to as SP (strip Packaging).
It is the use of two layers of pharmaceutical strip packaging film (SP) film to sandwich the tablets in the middle, a certain distance between the units of drugs, the two layers of SP film around the drugs in the strip packaging machine heat seal the inner side, the drugs between the pressure on the teeth, the formation of a unit of packaging forms (single tablet packaging or rows of small packages).
The strip pack is a continuous operation on the strip packer and is particularly suitable for automatic packaging of large quantities. When taking the medicine, the SP film can be torn along the teeth marks. The strip packaging film, with a certain tensile strength and elongation, is suitable for medicines of various shapes and sizes, and is packed close to the medicine inside and is not easy to produce ruptures and wrinkles.
More commonly used is aluminium-plastic composite film, such as cellophane and aluminium foil and polyethylene composite (PT/AL/PE), polyester and aluminium foil and polyethylene composite (PET/AL/PE), that is, aluminium foil and plastic film with adhesive lamination or extrusion composite, from the grass-roots level, printing layer, high barrier layer, sealing layer, grass-roots level outside, heat sealing layer inside, high barrier layer, printing layer in the middle.
The base material is required to have excellent mechanical properties, good glossy printing, good transparency, high barrier properties, safe and non-toxic, and no heat sealing. Typical materials are polyester (PET), cellophane (PT) and cellophane with PVDC coating.
The high barrier layer should have good gas barrier properties and be moisture resistant, impervious to bacteria and micro-organisms, have good mechanical properties, have a certain elongation, be cold and heat resistant, safe and non-toxic.
However, this soft aluminium foil is not transparent, does not rust itself and has a strong light-shielding effect.
If you need a transparent strip packaging film, it is necessary to use PVDC as a high barrier layer of material. PVDC as a high barrier layer of material, its most important feature is the gas, water vapour excellent barrier performance. The sealing layer is the inner layer of the strip packaging film, with excellent heat sealing, while having chemical stability and safety and hygiene, generally using low-density polyethylene material.
How to choose pharmaceutical packaging materials? Pharmaceutical companies choose what kind of pharmaceutical packaging with composite film, is to be determined by the nature of the drug (moisture, oxidation, taste retention, etc.) and shelf life, mainly according to the quality characteristics of the drug requirements, combined with the characteristics of the composite product selection for use, the following provides several pharmaceutical composite film characteristics for reference:
Ordinary composite film: composite structure to polyester and aluminium foil and polyethylene composite (PET/AL/PE), characterised by good printability, good gas and moisture barrier to drugs.
Pharmaceutical strip easy-tear laminate film: laminated structure with cellophane and polyethylene laminated with aluminium foil (PT/PE/AL/PE).
Features: good tearability for easy access to the product by the consumer. Good gas and water vapour barrier to ensure a long shelf life of the contents.
Suitable for the packaging of pharmaceutical products such as effervescent, coated and tablet capsules.
Paper-aluminium-plastic composite film: composite structure with paper and polyethylene laminated with aluminium foil (paper/PE/AL/PE), characterised by: good printability and good stiffness. It has good barrier properties to gas or water and can guarantee a long shelf life of the drug.
Don't forget to share this post!
Blister Packaging Machine Related Posts
Blister Packaging Machine Related Products
Blister Packaging Machine Related Videos
CONTACT US
Tell us your raw material and project budget to get quotations within 24 hours.
WhatsApp Us: +86 189 7157 0951
Want the best price & newest pharmaceutical machinery buying guide,tips and trends sent straightly to your box?Sign up for Aipak’s monthly newsletter,we’re free for your consultation and Offer you the most suitable solutions!
The Buyer's Guide
- Capsule Filling Buyer's Guide
- Blister Packaging Buyer's Guide
- Tablet Counting Buyer's Guide
- Tube Filling Buyer's Guide
- Cartoning Buyer's Guide
- Gummy Making Buyer's Guide
- CO2 Extraction Buyer's Guide
- Empty Capsules Buyer's Guide
- Suppository Filling Buyer's Guide
- Tablet Coating Buyer's Guide
- Tablet Press Buyer's Guide
- Softgel Encapsulation Buyer's Guide
Most Popular
- 7 Importance Of Pharmaceutical Packaging In Different Applications You Must Know
- 6 Advantages You Must Know About Tablet Counting Machine
- 8 Advantages of Blister Packaging You Must Know
- 6 Critical Applications of Automatic Capsule Filling Machine
- 6 Stations You must Know to Improve the Filling Quality of Automatic Capsule Filling Machine