Vietnam CIP In Pharmaceutical Industry The Complete FAQ Guide In 2025
As Vietnam's society and economy develop, more and more processing plants have made ensuring food and drug safety their primary goal. To achieve this goal, on-site cleaning is an important tool to achieve this goal. A series of CIP systems in pharmaceutical industry and food, dairy, beverage and other industries have emerged.
The Vietnam CIP system and equipment helps control, monitor and record hygiene. And it can make cleaning technology more efficient and economical, and ensure product safety. Let's take a look at Vietnam CIP in pharmaceutical industry.
1.What Is Vietnam CIP In Pharmaceutical Industry?

What Is Vietnam CIP In Pharmaceutical Industry-sourced: csidesigns
The CIP stands for cleaning in place. It is a professional system that is used to clean pharmaceutical production equipment without disassembly. The purpose of this method is to ensure that the drugs are not contaminated. It is a fixed or mobile device.
The Vietnam CIP is specially applied in Vietnam pharmaceutical industry. It can be used to clean pharmaceutical containers, inner surfaces of pipes, filters, process equipment and accessories without disassembly. This process is usually carried out in pipes, hoppers, tanks or water tanks to eliminate traces of bacteria or powder deposits.
2.Why The Vietnam CIP Is Important In Pharmaceutical Industry?
Why The Vietnam CIP Is Important In Pharmaceutical Industry-sourced: zwirnerequipment
The Vietnam CIP is really important in pharmaceutical industry. Because it helps to ensure that medicines are free of contaminants. In Vietnam pharmaceutical industry and process, if not strictly controlled, medicines may easily be contaminated by dirt, dust and microorganisms, which can affect the health of the person taking it and the patient.
3.What Are The Areas Application of Vietnam CIP In Pharmaceutical Industry?
The Vietnam CIP is mainly applied in pharmaceutical industry. At the same time, it can also be applied in other industries, including:
Pharmaceutical Industry
Pharmaceutical Industry-sourced: ag5
Pharmaceutical industry usually use the highest level of CIP technology. Because the hygiene standards of the pharmaceutical industry are very strict. In addition to the common high flow and strong spray, the pharmaceutical industry also adds specific pumps, valves and accessories to achieve the highest level of cleanliness.
Healthcare Industry
Healthcare Industry-sourced: micetcraft
Hospitals use CIP systems to sterilize surgical instruments and medical devices to prevent the spread of infection. This ensures cleanliness and sterility between production batches, maintaining product safety and quality.
Biotechnology Industry
Biotechnology Industry-sourced: gmpsop
Many biotechnology industries require a safe and sterile environment. Through Vietnam CIP technology, it can provide a sterile environment for laboratory experimental equipment and prevent biological samples from being contaminated.
Food Processing Industry
Food Processing Industry-sourced: cybernetik
Due to the outstanding efficiency of CIP systems, many breweries, milk and dairy factories use CIP to clean brewing containers and piping systems to ensure product quality.
4.What Do Vietnam CIP Clean and Sanitize In Pharmaceutical Industry?
The CIP system in pharmaceutical industry can clean internal product contact surfaces (such as process pipes, containers and equipment) without disassembling the equipment. Its main application products include:
Vessels and Tanks
Vessels and Tanks-sourced: gpi-pharmaceutical
Especially for tanks containing dairy products, it is very easy to breed bacteria. The CIP system can remove milk scale without dead corners and sterilize it by pasteurization.
Pipes
Pipes-sourced: sanitaryfittings
Pipes in pharmaceutical manufacturing are essential for transporting drugs, purified water, steam, compressed air, nitrogen, pharmaceutical solutions, etc. Pipeline design is an integral part of pharmaceutical plant design.
Fillers
AIPAK Fillers
There are multiple fillers for pharmaceutical manufacturing, including liquid, syrup, ampoule, powder, paste, cream, etc.
Mixers
AIPAK Mixers
There are plenty of mixers that are commonly used in pharmaceutical industry for mixing substances with different viscosities and densities efficiently, including high shear mixer, ribbon mixers, double cone mixers, v-mixers, homogenizers, etc.
5.What Are The Benefits of Vietnam CIP In Pharmaceutical Industry?
The Vietnam CIP in pharmaceutical industry can bring many advantages to your integrated equipment. These include:
Prevent Contamination
Prevent Contamination-sourced: csidesigns
CIP in pharmaceutical industry ensures that all processing equipment and piping systems are thoroughly cleaned and disinfected after each batch of product production runs. This prevents the accumulation of contaminants such as bacteria, viruses and other harmful microorganisms in the workplace and affects drug safety.
Prevent Drug Cross Contamination
Prevent Drug Cross Contamination-sourced: bioprocessintl
In addition to preventing drugs from being contaminated by bacteria, viruses and other harmful microorganisms during the production process, it can also avoid cross contamination of allergens, raw foods and drug ingredients in drugs, thereby improving product quality.
Improve Labor Safety
Improve Labor Safety-sourced: processingmagazine
The CIP system requires less labor work, thereby reducing the potential risk of workers being exposed to hazardous cleaning chemicals, and eliminates human error, ensuring safety during pharmaceutical production.
Save Time and Labor Costs
Save Time and Labor Costs-sourced: gravityflow
CIP in pharmaceutical industry mainly uses automated cleaning systems, which do not require manual cleaning, saving both effort and time. In addition to saving time and labor costs, it also ensures that the cleaning process is consistent and effective.
Reduce the Use of Water and Chemicals
Reduce the Use of Water and Chemicals-sourced: researchgate
During the use of CIP system, the use and dependence of water and chemicals will be reduced, and the impact on the environment and operating costs will be reduced.
Reduce Downtime
Reduce Downtime-sourced: collabitsoftware
The CIP system mainly uses a fully automatic cleaning system without disassembly, which reduces the downtime for equipment cleaning, maintenance and repair. This system is more energy-efficient and uses less water and cleaning chemicals.
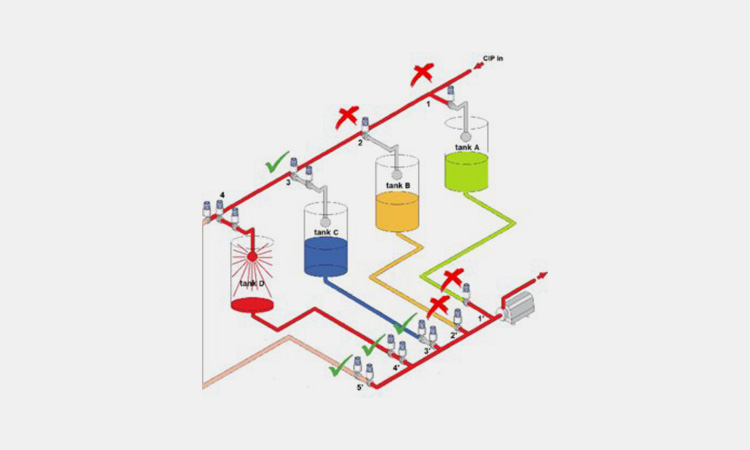
6.How The Vietnam CIP Works In Pharmaceutical Industry?
How The Vietnam CIP Works In Pharmaceutical Industry-sourced: fluidhandlingpro
The Vietnam CIP works in a simple and organized way in the pharmaceutical industry. It delivers a series of cleaning solutions to the equipment being cleaned. This helps remove contaminants from the surface of the equipment. The CIP spray system then rinses the equipment with water to remove any residual cleaning solution.
7.What Are The Agents Usually Used In Vietnam CIP In Pharmaceutical Industry?
The Vietnam CIP utilizes formulated cleaning agents to wet, dissolve, saponify, emulsify, disperse and isolate contaminants. The types of cleaning agents frequently used in CIP include:
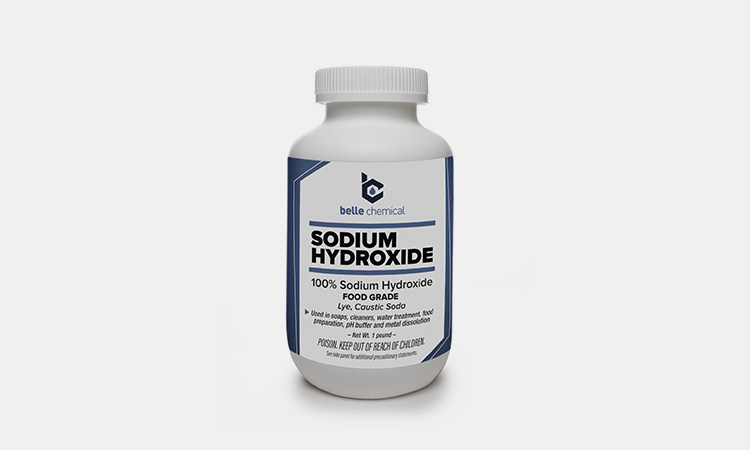
Sodium Hydroxide
Sodium Hydroxide-sourced: bellechemical
Sodium hydroxide removes contaminating proteins and nucleic acids while inhibiting the growth of different bacteria, viruses, fungi, yeasts and endotoxins. It is mainly used for contaminants in pharmaceutical production environments.
Nitric Acid and Phosphoric Acid
Nitric Acid and Phosphoric Acid-sourced: fivestarchemicals
Nitric Acid and Phosphoric Acid are mainly used with highly oily equipment after alkaline cleaning. It stabilizes the pH after alkaline cleaning and does not leave any significant residue.
Peracetic Acid (PAA)
Peracetic Acid (PAA)-sourced: murphyandson
Peracetic Acid is an oxidizing agent. It is able to eliminate organic matter such as gram-negative and gram-positive bacteria, yeast, fungi, etc. It is the preferred cleaning agent for CIP because it does not leave harmful residues and is effective even at low temperatures.
Sodium Hypochlorite
Sodium Hypochlorite-sourced: shopmazo
Sodium Hypochlorite has high antimicrobial properties and is widely used in CIP practices. It effectively removes dry and fixed organisms and biofilms on surfaces. Its advantages are that it is cheap, is not affected by water hardness and does not leave harmful residues.
Chlorine Dioxide
Chlorine Dioxide-sourced: clearspa
Chlorine dioxide is often used as a substitute for hypochlorite. It is effective against high organic pollutants. The advantage of this substance is that it is less corrosive and less harmful to the environment.
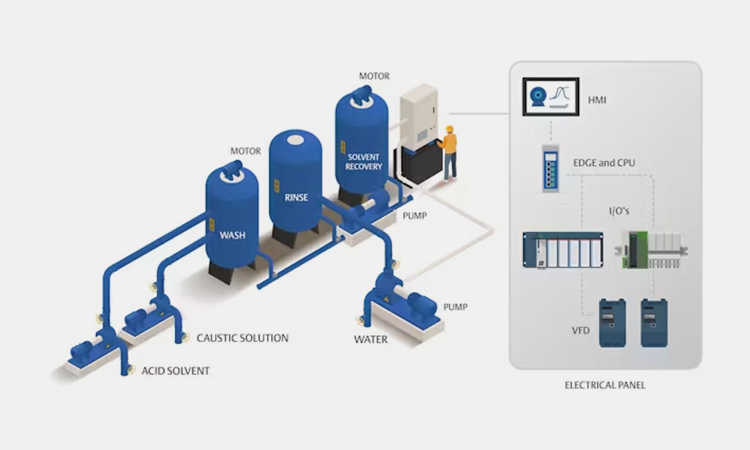
8.What Are The Vietnam CIP Process In Pharmaceutical Industry?
The most common steps of the CIP process in the pharmaceutical industry are usually as follows:
Vietnam CIP Process In Pharmaceutical Industry-sourced: burkertpacific
Pre-rinse. Remove water, air and residues from the equipment and pipes, thereby reducing the amount of cleaning products and wastewater;
Caustic Wash. Remove fats and more corrosion-resistant product residues from the equipment;
Intermediate Rinse. Use re-circulation to remove traces of residues and alkaline solutions from the equipment or tank body;
Acid Wash. Break down calcium and other deposits on the equipment or tank walls;
Final Rinse. Remove residual acid solutions and detergent residues. And use chemicals, ozone, hot water or steam for sterilization cycles.
9.What Are The Components of a Vietnam CIP In Pharmaceutical Industry?
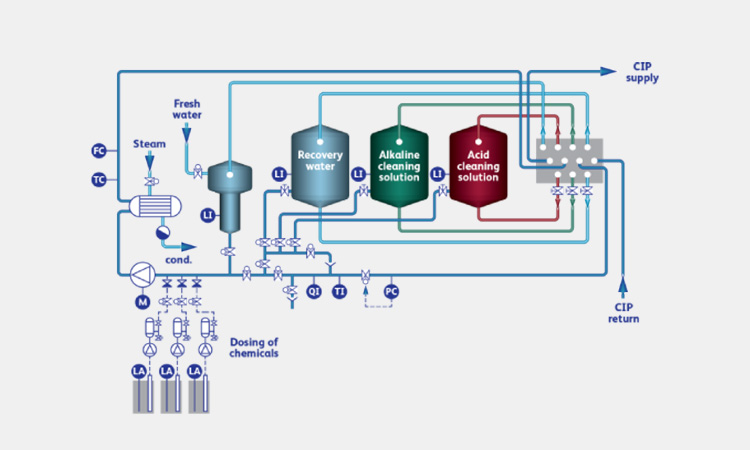
Components of a Vietnam CIP In Pharmaceutical Industry-sourced: be-machinery
The main components of a CIP system include:
Cleaning Agent Tank
A typical CIP system will have 3-5 tanks for rinse water and different agents. They contain detergent solutions prepared according to the concentration used. One tank contains an alkaline solution to dissolve proteins and saponify grease. One tank contains an acid solution to dissolve lime and rust.
Cleaning Agent Supply Valve
The cleaning agent supply valve is mainly used to release and control the amount of water and detergent in the equipment cleaning cycle. Different supply valves are suitable for different detergents.
Cleaning Agent Return Valve
The return valve prevents the back-flow of detergents and liquids in different tanks. It also prevents sewage from flowing into the water supply system, providing safety for your cleaning.
Heat Ex-changers
The heat exchanger is the heating system of the tank. It is a control system with temperature, liquid level and conductivity sensors. Through the pump group and valve group, the cleaning equipment is circulated.
Control Cabinet
The control cabinet is the core component of the CIP system. It controls all filtering, preheating, mixing and pumping of water, detergent and deionized water. And monitors cleaning data, including flow rate, detergent concentration, temperature and cleaning time.
Steam Entrance
The steam entrance is mainly responsible for the final sterilization cycle. When all residues and detergents have been cleaned up, the Steam Entrance will automatically deliver circulating sterilization steam to disinfect and sterilize the entire equipment.
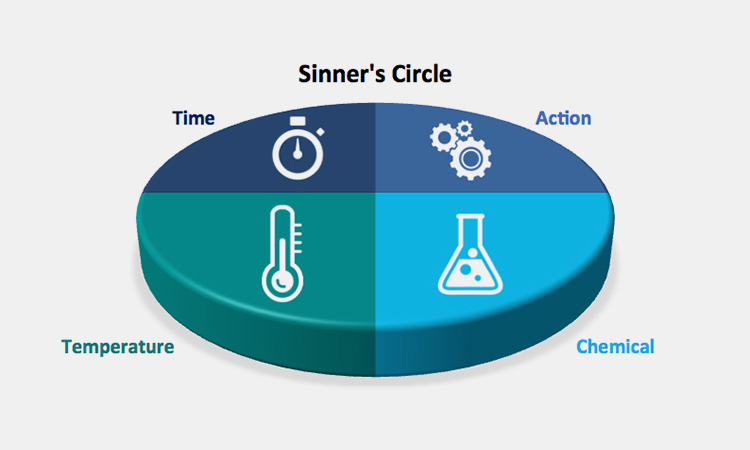
10.What Are The Operational Requirements For Vietnam CIP In Pharmaceutical Industry?
Learning the operational requirements for Vietnam CIP in pharmaceutical industry is essential for the operation of the CIP system. Next, you need to learn to control and record:
Operational Requirements For Vietnam CIP In Pharmaceutical Industry-sourced: sanimatic
Record Temperature
Learning to record temperature is a key step in the entire process. In the CIP system, the temperature of the solution is mainly determined by the process requirements and the activity of the cleaning chemical. Too high will damage the equipment and the activity of the cleaning agent, and too low will cause residues and incomplete cleaning.
Maintain Water Flow Rate
Maintain Water Flow Rate-sourced: oem
The key factor in the cleaning effect of the CIP system is to maintain turbulence in the pipeline, that is, the "scrubbing" of the water force. Try to control the flow rate of the water flushing to about 1.5 m/s to achieve a good cleaning effect.
Record Pressure
Record Pressure-sourced: hamiltoncompany
In the CIP system, sufficient pressure is very important for the reliable performance of the spray device. The pressure of the CIP solution is proportional to the square of its velocity. Therefore, recording the water flow pressure is crucial.
Control the Concentration of Detergent
The detergent of the CIP system is mainly a disinfectant or bactericide, that is, an acid or an alkali. The chemical concentration of the detergent is an important indicator of the effectiveness of the cleaning. You can measure manually or automatically with a conductivity probe.
Cleaning Time
The contact time between the cleaning solution and the surface being cleaned is also critical, given all the conditions, including temperature, flow, pressure, concentration. This includes the amount and nature of the fouling, surface geometry, chemical concentration and cost, pipeline availability, etc.
11.What Are The System Validation For Vietnam CIP In Pharmaceutical Industry?
How to verify the cleaning effect of Vietnam CIP in pharmaceutical industry? What acceptance criteria need to be met to be qualified? The following acceptance criteria can answer your doubts.
Visual Inspection
Visual Inspection-sourced: sterislifesciences
Visual inspection is one of the quickest and easiest methods. For single-use equipment without cross-contamination problems, visual inspection can be used to check whether the equipment has residues or cleaning agents.
Equipment Sampling
Equipment Sampling-sourced: sanimatic
By swabbing the surface of the equipment, and then extracting and analyzing the residues in the swab to determine whether your CIP system's cleaning work has met the standards. Among them, the sample must represent the worst case of the entire system.
Wastewater Sampling
By sampling the wastewater after the CIP system is cleaned separately, the contaminant analysis is performed. The residual value of the active substance in the placebo in the wastewater contaminant is analyzed for verification. This method is very effective for food processing systems.
Perform Analysis
Perform Analysis-sourced: pulse
By analyzing the residues in the equipment swab and wastewater sampling, the detection limit needs to be less than 25%.
12.What Are The Considerations Before Owning The Vietnam CIP In Pharmaceutical Industry?
If you are considering an ideal Vietnam CIP in pharmaceutical industry, you need to consider the following factors:
Identify Your Cleaning Needs
Identify Your Cleaning Needs-sourced: socure
Based on your own products, equipment and specific regulatory requirements, determine the equipment and components you will clean, the frequency of equipment cleaning, the cleaning agents and concentration requirements, and the time of cleaning.
Identify Your Production Plan
Identify Your Production Plan-sourced: chinabrewingequipment
In addition to clarifying your cleaning needs, in order to meet your cleaning efficiency as much as possible, you need to design your production plan to meet your cleaning needs as efficiently as possible. This includes checking the size, cleaning frequency, cleaning method and flow rate of your production system.
Determine Available Space
Determine Available Space-sourced: cybernetik
Due to the limited space and complexity of the system, when considering a CIP system, you need to arrange the appropriate CIP system according to your own space constraints. If there is enough space, you can consider using a single tank to hold the cleaning solution and centrally operate different circuits of each pipeline.
Calculate the Amount of Chemicals and Water Used
The biggest benefit of an automated CIP system is the ability to intelligently control the amount of cleaning agents, water and chemicals used. Reducing the use of chemicals can reduce the pressure on the environment, while also reducing cleaning time and optimizing the disinfection process.
Ensure CIP System and Equipment Layout Compatible
Some product manufacturing processes or manufacturing equipment or even drainage systems are not compatible with the CIP system. Therefore, you need to check the system configuration of the production equipment in advance to avoid unnecessary damage.
Conclusion:
Installing a CIP system can bring you huge economic benefits, maximizing production quality and minimizing other activities and costs. However, the larger and more difficult the cleaning work is, the more cost-effective CIP will be. Through this Vietnam CIP in pharmaceutical industry, you may get their details. If there are more you want to know, contact us now!
Don't forget to share this post!
Vial Filling Machine Related Posts
Vial Filling Machine Related Products
Vial Filling Machine Related Videos
CONTACT US
Tell us your raw material and project budget to get quotations within 24 hours.
WhatsApp Us: +86 181 6426 8586
Want the best price & newest pharmaceutical machinery buying guide,tips and trends sent straightly to your box?Sign up for AIPAK’s monthly newsletter,we’re free for your consultation and Offer you the most suitable solutions!
The Buyer's Guide
- Capsule Filling Buyer's Guide
- Blister Packaging Buyer's Guide
- Tablet Counting Buyer's Guide
- Tube Filling Buyer's Guide
- Cartoning Buyer's Guide
- Gummy Making Buyer's Guide
- CO2 Extraction Buyer's Guide
- Empty Capsules Buyer's Guide
- Suppository Filling Buyer's Guide
- Tablet Coating Buyer's Guide
- Tablet Press Buyer's Guide
- Softgel Encapsulation Buyer's Guide
Most Popular
- 7 Importance Of Pharmaceutical Packaging In Different Applications You Must Know
- 6 Advantages You Must Know About Tablet Counting Machine
- 8 Advantages of Blister Packaging You Must Know
- 6 Critical Applications of Automatic Capsule Filling Machine
- 6 Stations You must Know to Improve the Filling Quality of Automatic Capsule Filling Machine