Manufacturing Of Semi-Solid Drug Products: The Complete Buying Guide In 2025
Have you ever wondered about that antibiotic ointment you use to treat your wound infections comes to you after going through an established manufacturing process? There are many products, either analgesic gel you applied few days back for knee pain or cream prescribed by your doctor for your skin, all require efficient and high quality manufacturing to give their impactful effects.
If you are a buyer, and looking forward for a fruitful investment, you do not want to miss any chance to make investment in manufacturing of semi-solid drug products due to its commanding role in society. This topic is designed to guide you to make a well-informed decision in this regard.
1.What do you understand from manufacturing of semi-solid drug products?
Antibiotic Ointment-Picture Courtesy: Scientific American
What idea comes to your mind when you read the word semi-solid? It’s like something that is half solid and half liquid. Well you are right then. Manufacturing of semi-solid drug products relates to the production of such drug items that’s consistency lies between solid and liquid. These formulations include ointments, creams, gels, pastes and suppositories.
All these semi-solid drug products have great importance in our lives, so its manufacturing possesses the same place. As, efficient manufacturing processes give you an effective product. As a purchaser, your interest gets tripled after knowing about its impactful role.
2.What are the types of semi-solid drug products?
There are many types of semi-solid drug products, do you know what are they?
Ointment:
An Ointment Used To Treat Skin Disease
Ointment is semi-solid drug product, which is greasy, thick and hydrating by nature. It is used to treat skin, eye and mucous membrane ailments and applied topically.
Examples:
Hydrocortisone ointment is used to treat skin inflammation and allergies.
Neomycin is used to treat skin wound infections.
Cream:
An analgesic cream
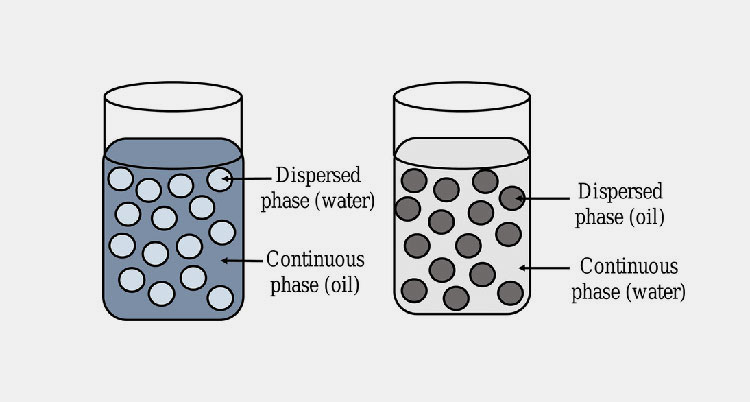
Cream is the semi-solid drug product, which is emulsion of oil and water and emulsifying agent is incorporated to mix these two phases. It is  less greasy in nature than ointments and has good spread ability. It is available in to two types oil-in-water and water-in-oil creams.
Examples:
Diclofenac cream is used to relief pain related to muscles and joints.
Ketoconazole cream is used to treat fungal infections.
Gel:
A Pain Relief Gel
Gel is semi solid drug product, which can be transparent or translucent. It is non-greasy and hydrophilic in nature as gelling agent is added to make this drug product. It offers abundant cooling effect and fast drug release.
Examples:
Diclofenac gel is used to treat pain of joints and muscles especially related to arthritis.
Aloe vera gel is used to treat skin burns and act as hydrating agent.
Paste:
Zinc & Salicylic Acid Containing Paste
Paste is semi-solid drug product, very thick in consistency as contains excess amount of solid content. It offers good local effect and protective barrier to skin.
Examples:
Magnesium sulfate paste is used to treat rashes and bed sores.
Salicylic acid paste is used to treat corns and calluses.
Lotion:
Clindamycin Lotion For Acne
Lotion is semi-solid drug product. It is basically an emulsion or a suspension, less thick in consistency and has very good spread ability over a vast area of skin. It provides cooling effect to skin and evaporates rapidly.
Examples:
Calamine lotion is used to relief itching.
Clindamycin lotion is used to treat acne.
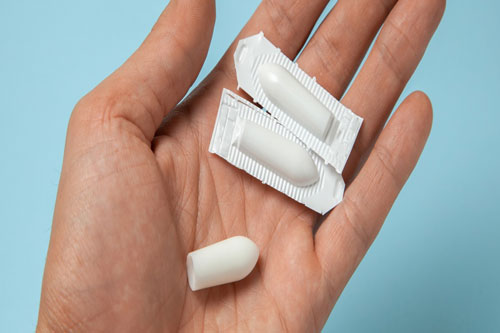
Suppository:
Paracetamol Suppository Act As Anti-Pyretic And Analgesic
Suppository is semi-solid drug product, cooled and shaped before packaging in order to provide ease in administration. It is used for vaginal or rectal drug delivery and it gets melt at body temperature.
Examples:
Glycerin suppository is used for treatment of constipation.
Paracetamol suppository is used to treat fever.
Miconazole suppository is used to treat vaginal fungal infection.
3.What is the advantage of manufacturing of semi-solid drug products to the pharmaceutical industry?
So many drug products which are making our lives easy are semi-solid by nature like ointments, gels, creams, pastes and suppositories. All these drug products are manufactured with great efficiency and precision in order to provide their profound effects. This topic sheds light about the profitable role of manufacturing of semi-solid drug products.
Establish stability of drug:
Sensitive Drug Like Antifungal Is Dispensed In Form Of Cream To Keep Stability Intact-Picture Courtesy: McKesson Medical Surgical
Stability of sensitive natured drugs (like NSAIDs, antibiotics & antifungal) is established by the help of manufacturing of semi-solid drug products. In this way you get their beneficial effects whenever you need.
Support patient compliance:
Semi-Solid Drug Products Are Easy To Apply-Picture Courtesy: SkinKraft
Various semi-solid drug products are easy to apply and provide effective localized and systemic effects. Manufacturing of semi-solid drug products reduces the need of frequent dosing and invasive procedures, in this way it supports patient compliance.
Selected drug delivery:
Semi-Solid Drug Products Provide Localized Effects-Picture Courtesy: Verywell Health
Semi-solid drug products provide localized effects like for eyes, skin, mucous membrane, joints, muscles etc., avoiding the unpleasant systemic effects and enhancing therapeutic efficacy. In this way manufacturing of semi-solid drug products is crucial for pharmaceutical industry.
Versatile drug product:
Above Skin Ointment Contains Polymyxin B, Bacitracin And Lignocaine Together To Provide Profound Effect
Manufacturing of semi-solid drug product offers such formulation that can include different active pharmaceutical ingredients (API) in it, that possesses distinct solubility and stability characteristics.
Offer advancement in drug delivery system:
Controlled Release Skin Emulsion-Picture Courtesy: Reddit
Manufacturing of semi-solid drug products presents such formulations that are hydrogen based or liposomal systems. These formulations offer controlled or sustained drug release enhancing the efficacy of drug.
Cost effective manufacturing:
Manufacturing Of Semi-Solid Drug Products Is More Cost Effective As Compared To Solid Drug Products
Manufacturing of semi-solid drug products is less complicated and demands moderate investment in technical equipment as compared to solid drug products like tablets and capsules.
Vast area of application:
Applying Eye Ointment-Picture Courtesy: Cats.com
Manufacturing of semi-solid drug product covers a vast area of application. These products are utilized in dermatology, ophthalmology, gynecology, rectal and trans-mucosal drug delivery system.
4.What raw materials are utilized in manufacturing of semi-solid drug products?
Raw materials are crucial part of any manufacturing and as a purchaser you must have information about it in order to get an impactful outcome. Manufacturing of semi-solid drug products like gels, ointments, creams, pastes and suppositories needs a blend of various raw materials to acquire the expected uniformity, stability and drug delivery characteristics.
| Raw materials | Pictures | Examples |
| Active pharmaceutical ingredients (APIs): these are the therapeutically active agents accountable for the intentional effects of drug. | 
Ketoconazole Used As Active Pharmaceutical Ingredient |
Antibiotics: Bacitracin, polymyxin.
Anti-inflammatory drugs: diclofenac. Antifungal: ketoconazole Analgesic: lidocaine |
| Bases: these are act as vehicle or carrier and play its role in forming bulk of drug product. | 
Paraffin-Picture Courtesy: Chemical Store Inc |
Hydrocarbon bases: used as an ingredient in ointments for occlusive effects. E.g. petroleum jelly, paraffin.
Water-soluble bases: used as an ingredient in gels. E.g. polyethylene glycol, carbopol. Emulsion bases: used as an ingredient in creams. E.g. cetostearyl alcohol, emulsifying wax. |
| Surfactants: they have a vital role of mixing water and oil phases to form creams or lotions | 
Sodium Lauryl Sulfate-Picture Courtesy: Trunnano |
Anionic surfactants: sodium lauryl sulfate.
Non-ionic surfactants: polysorbates. Cationic surfactants: benzalkonium chloride. |
| Gelling agent: it is specific for gels and pastes, has role in giving gel-like consistency and controlling viscosity. | 
Tragacanth-Picture Courtesy: Britannica |
Natural polymers: tragacanth, gelatin.
Synthetic polymers: hydroxylpropyl methylcellulose. Clays: magnesium aluminum silicate. |
| Humidifying agent: it has role in prevention of dryness and keeping formulation wet. | 
Glycerin-Picture Courtesy: Anveya |
Glycerin.
Propylene glycol. Sorbitol. |
| Preservatives: they have role in preventing from microbial growth and expanding shelf life of drug product. | 
Methyl Paraben |
Phenoxyethanol.
Methylparaben. Benzyl alcohol. |
| Stabilizers and antioxidants: aid in avoidance of oxidation process and destruction in drug product. | 
Ascorbic Acid-Picture Courtesy: SD Precise Bioscience |
Ascorbic acid (vitamin C).
Tocopherol (vitamin E). |
| pH modifiers: help to keep the stability and compatibility of drug product. | 
Citric Acid-Picture Courtesy: Health |
Citric acid.
Lactic acid. |
| Penetration enhancer: helps in drug penetration through the skin. | 
Dimethyl Sulfoxide-Picture Courtesy: Lab Valley |
Dimethyl sulfoxide.
Oleic acid. Ethanol. |
5.What kind of packaging containers is used in manufacturing of semi solid drug products?
The benefits, we are experiencing every day from manufacturing of semi-solid drug products are generous. But have you ever thought that packaging container has its role too in delivering such advantageous product to us. For your knowledge, packaging container has vital role in shielding the formulation from damage-causing factors so that you could receive impactful effects. Choice of container depends upon characteristic of formulation.
| Packaging containers | Information |
| Tube
Ophthalmic Ointment |
Most commonly used packaging container in manufacturing of semi-solid drug products.
This is made up of aluminum or laminated plastics. Creams, gels, pastes and ointments are dispensed in it. Benefits of this packaging container can be: It provides efficient protection from any possible contamination. It facilitates controlled dispensing. It is easy to carry due to having less weight. |
| Jar
Magnesium Sulfate Paste |
Many semi-solid drug products are packaged in jar like balm, pastes, thick creams etc.
It is usually made up of glass or plastic (PET). Advantage of jar includes convenience to get large quantities of formulation so, appropriate for semi-solid drug products that are applied manually. |
| Bottle
Pain Relief Gel |
Bottle is mostly used for less viscous semi-solid drug products.
Typically made up of plastic (PET) or glass. Gels or emulsions are dispensed in it. Benefits include convenient to use and offer controlled dispensing by incorporating flip-top caps or pump dispensers. |
| Aluminum or plastic packaging for suppositories
Suppositories-Picture Courtesy: Health Central |
Suppositories are dispensed sealed packaging, made up of aluminum, plastics or laminates
Benefits are having little weight, easy to use and offer good protection to formulation. |
6.What are the stages of manufacturing of semi-solid drug products?
As a purchaser, it becomes integral to know about stages of manufacturing of semi-solid drug product. This knowledge helps you to get wholesome benefits from this manufacturing. Several important stages are implicated in manufacturing to assure the stability, durability and efficacy of formulation. Stages are discussed below:
Sourcing of raw material and formulation development:
Pre-Formulation Evaluation-Picture Courtesy: UPM Pharmaceuticals
Precise measurement of raw materials is practiced according to the composition of formulation. To examine different aspects before going to formulate drug product is essential. This evaluation helps you to attain desired quality drug product. First of all selection of active pharmaceutical ingredients and excipients is carried out to achieve intentional drug effects.
Compatibility among ingredients is integral, studies are carried out to make sure about this. Establishment of demanded physiochemical characteristics like viscosity, spread ability etc. is necessary as this decides about characteristics of drug products.
Oil In Water / Water In Oil Emulsion-Picture Courtesy: ReseachGate
Choosing base for your semi-solid drug product is initial step; it can be oil-in-water / water-in-oil emulsion or gel). Upgrading the ingredients for their stability, efficacy and skin penetration is very important. Laboratory tests are carried out to decide the composition of your formulation.
Mixing and homogenization of ingredients:
Homogenization Of Ingredients
Mixing of active pharmaceutical ingredients and excipients is performed by the help of mixers or homogenizers.
Addition of surfactants (if needed):Surfactants are added in creams and lotions as emulsifying agents. They play role in formation of stable emulsion. After addition of surfactant, homogenizer is utilized to get uniform droplet size and stable formulation.
Surfactants Are Necessarily Added In Creams To Attain Stability
Addition of gelling agents (when needed): Gelling agents are incorporated in gels and pastes to make them viscous.
Methyl Cellulose, A Gelling Agent-Picture Courtesy: Reddit
Addition of pH modifiers: pH is adjusted according to skin competency by the aid of pH modifiers.
Lactic Acid As pH Modifier-Picture Courtesy: DermaGen By Botanical Chemist
Quality control:
Different Tests Are Carried Out To Yield High Quality-Picture Courtesy: Antidote.me
To attain high quality of semi-solid drug products, different test are performed, which are listed below:
| Tests | Purpose of testing |
| Physical tests: | Various physical tests are performed to check the viscosity, spread ability and uniformity of semi-solid drug products. |
| Chemical tests: | pH, drug quantity and purity is evaluated through chemical testing. |
| Microbiological tests: | Sterility and preservative effectiveness is checked by the help of microbiological tests. |
| Stability test: | Evaluation of the strength of drug product to remain stable in different conditions is done by stability test. |
Packaging and labeling:
Aluminum Tubes-Picture Courtesy: Pharmaceutical Tubes
The furnished semi-solid formulation is filled in to suitable containers like tubes, jars or sachets. After the completion of filling process, next step is to seal the container appropriately to nullify the chance of contamination and to keep the stability of semi-solid drug product intact. Label the final drug product with information about how to use it, expiry date and batch number.
Storage:
Monitoring Of Temperature And Humidity In Storage Area Is Integral-Picture Courtesy: G&S Mechanical
Storage of semi-solid drug products is carried out under controlled temperature and humidity condition to maintain the quality.
7.What machines are involved in manufacturing of semi-solid drug products?
Manufacturing of semi-solid drug products requires efficient machines to ensure stable, durable and potent drug product. Before making an investment in this kind of manufacturing, it becomes necessarily important to gain details of machines utilize in it. This topic is designed to make you aware about important machines participating in manufacturing of semi-solid drug products.
Mixing and homogenization machines:
| Machines: | Purpose: |
| Vacuum emulsifier mixer
AIPAK Vacuum Emulsifier Mixer |
It is used to form smooth semi-solid drug products by providing uniform blending and eliminating air bubbles from formulation. |
Heating and cooling machines:
| Machines: | Purpose: |
| Jacketed tanks
Picture Courtesy: Pharmaceutical Storage Tanks |
Utilized for heating or cooling of ingredients during process of mixing. |
| Heat exchangers
|
Used to perform cooling processes without causing any contamination to drug product and maintaining stability. |
Filling and packaging machines:
| Machines: | Purpose: |
| Tube filling and sealing machine
AIPAK Tube Filling And Sealing Machine |
It is utilized in filling and sealing semi-solid drug product in to plastic or aluminum tubes.
Tubes of desirable size and material are loaded in tube loader; on the other hand semi-solid drug product is subjected in to the hopper. As the process starts with supply of tubes to filling station, accurate amount of semi-solid drug product is filled in tubes by the help of filling nozzles then tubes are moved further to get sealed in sealing station according to the requirement. |
| Lotion filling line
Lotion Filling Line |
It is intended to use for filling semi-solid drug products in to bottles or jar.
Firstly, bottles/ jars are washed and then dried. After that filling process is carried out, and then capping is done by the same equipment. |
| Suppository filling production line
Picture courtesy: AIPAK suppository filling production line |
It is specially designed to fill rectal or vaginal suppositories.
First of all semi solid formulation is cooled then cut it in demanded shape like bullet-head, torpedo shapes etc. After that seal the formulation and dispense it in aluminum or plastic packaging. |
| Labeling machine
Picture Courtesy: Accent Label Automation |
This machine is used to apply labels on containers of finalized semi solid drug products.
Labeling machine can be fully-automatic, semi-automatic or manual. Labeling machine is chosen on the basis of packaging type like: Tubes are labeled by tube wrap-around labeler. Jars / bottles are labeled by the help of round bottle/jar labeler. Flat containers are labeled by flat surface label applicator. |
8.What are the key considerations for manufacturing of semi-solid drug products?
The complete process of manufacturing of semi-solid drug products like creams, ointments, gels etc. requires attentive control upon the formulation, processing and packaging to achieve a highly stable and top quality final product. There are various main factors that need to be considered for getting a successful outcome and as being a buyer you must know each of them.
Considerations about formulation:
API selection is an important factor to consider-picture courtesy: Netnews
Selection of active pharmaceutical ingredient (API) is an important factor and it needs to be done efficiently. API must have compatibility to the base it will be incorporated. Additionally, API must remain stable and soluble in recommended base.
Selection of base is another important thing. Base comprises of different excipients like emulsifiers, emollients, thickeners etc. so, choose such combination that provides suitable consistency and release profile.
Viscosity must be maintained according to the requirement of formulation as fine viscosity assures convenience in application, skin penetration and spread ability.
Stability must remain intact throughout the whole shelf life, its chemical, physical or microbiological. Every aspect is important.
pH modification in also needs to be consider for skin competency and drug stability.
Considerations about manufacturing process:
Optimal Temperature Must Be Kept During Manufacturing Process To Attain Stable Product-Picture Courtesy: Dreambox Learning
A very important aspect is uniform mixing and homogenization of API and excipients to get a smooth formulation that does not attain separation of phase.
Another important thing is process of heating and cooling. Temperature specification is essential to avoid any kind of degradation and attain a smooth texture. Below, temperature specifications are mentioned about different semi-solid drug products:
| Process | Temperature specification |
| Heating during mixing and melting process | Ointments are lipid based drug product, it can be melted or mixed in temperature 50-80 C.
Creams consist of two phases so oil and water phases can be heated at 60-75 C. After that both phases are mixed to get a uniform and stable drug product. In gels, polymer is used so its dissolution can take 40-80 C, according to the characteristic of gelling agent. |
| Cooling | Cooling done specifically at 25-40 C to keep formulation stable and smooth.
Fast cooling can result in phase separation. |
Some semi-solid drug products like creams demand optimal shear force and cooling rate to achieve stable droplet size.
Aseptic conditions are crucial for manufacturing of semi-solid drug products. So it must be maintained throughout the whole process.
Considerations about quality control:
Microbial Contamination Can Be Checked By Different Microbial Tests-Picture Courtesy: Nature
Microbial contamination must be avoided by the use of preservatives and maintaining aseptic conditions. Contamination can be checked by different microbial tests.
Particle size distribution has great influence on release of drug and texture uniformity.
All processes must follow guidelines defined by standard regulatory in order to achieve stable and durable product.
Considerations about packaging and storage:
Choose Appropriate Packaging Container For Your Formulation
Packaging type has significant role in keeping semi-solid drug product stable, so choose appropriate packaging container like tubes, jar etc. for your drug product that do not have interaction with formulation.
Shielding from light and air is necessary to keep semi-solid drug product stable, so utilization of opaque and air tight containers is made possible.
Temperature-sensitive formulations are stored on specific conditions; sometimes require refrigeration to ensure stability.
9.What are the failures of manufacturing of semi-solid drug products and how can you overcome them?
Maintaining The Stability And Quality Of Manufacturing Of Semi-Solid Drug Products Is Essential-Picture Courtesy: My Physio Perth
Maintaining the stability and quality of manufacturing of semi-solid drug products is essential; otherwise you can face failures which will influence the quality of formulation. This topic is crafted to educate you about expected failures reported, what were the causes and how would you overcome them. Such information is very crucial for a buyer and it will benefit you in future as well.
| Failures | Reasons | Solutions |
| Separation of phases in creams, emulsions etc.) | Inappropriate concentration of emulsifier.
Unsuitable speed of mixer or incorrect order of ingredient addition. Improper blending. Temperature is not specified. Stability is not attained due to pH changes. |
Utilize appropriate concentration of emulsifier.
Optimal shear rate must be applied during homogenization. Adjust pH according to demand of formulation. Temperature must be controlled according to requirements of formulation.
|
| Grittiness or particle clumping | Inappropriate dispersion of active pharmaceutical ingredients (APIs) and excipients.
Improper homogenization of ingredients. Insufficient dissolution of ingredients. |
Mixing or homogenization must be efficient.
Incorporate pre-dispersed APIs or fine particles in formulation. Use suitable surfactants or wetting agents. |
| Unsuitable viscosity (too dilute or too concentrated formulation) | Polymer concentration is incorrect.
Insufficient hydration or thickening agent dispersion. Uneven homogenization. Temperature is not specified. |
Use desirable amount of gelling agent or polymer.
Proper hydration and homogenization must be carried out. Perform viscosity test. Set optimal temperature. |
| Uneven mixing or homogenization: | Inconsistent temperature distribution.
Inappropriate mixing time or mixing rate. Equipment is not calibrated. |
Follow mixing guidelines properly.
Calibration of equipment must be done on daily basis. |
| Temperature based failures: | Excessive heating can destroy APIs or emulsifying agents.
Rapid cooling may result in crystallization |
Follow proper guidelines regarding temperature specification during heating and cooling and monitor temperature throughout the process. |
| Poor sealing: | Incorrect sealing methods.
Defective material of packaging. |
Calibrate sealing machine and use appropriate methods of sealing.
Use good quality packaging material. |
| Air bubbles present in formulation | Excessive speed mixing can produce air in formulation
Incorrect degassing before filling the formulation in container. |
Use vacuum mixers for formulations that can develop such defect.
Minimize the agitation speed prior to filling. |
| Microbial contamination | Poor hygiene in manufacturing area.
Water is not purified before use. Inappropriate amount of preservatives. |
Must follow cleaning protocols
Make sure to maintain aseptic environment. Water must be purified before use. Optimal amount of preservative must be used.
|
| Discoloration of formulation: | Oxidation of ingredients can cause discoloration.
Unstable pH. Exposure to heat and light. |
Incorporate anti-oxidants like ascorbic acid , tocopherol etc.
Use pH stabilizers. Use light-shielding packaging. |
| Failure in stability test | Poor storage conditions.
Unstable formulation. |
Perform stability studies.
Formulation must be re-made if needed to achieve desirable stability. |
10.What is the shelf life of manufacturing of semi-solid drug products and what parameters have their influence on their shelf life?
How many chief products we obtain from manufacturing of semi-solid drug product? Those are bringing comfort to our lives and healthcare system. But wait, these products must have an expiry date as similar to other drug products and you need to know about it too. This topic provides knowledge about shelf life of semi-solid drug products and what can influence their shelf life.
Shelf life of different semi-solid drug products:
| Semi-solid drug products | Shelf life |
| Creams & ointments | 1-3 years |
| Gels | 2-3 years |
| Lotions | 1-2 years |
| Suppositories | 1-3 years |
Parameters that can influence shelf life:
| Parameters | Their influence |
| Stability of active pharmaceutical ingredients (APIs)
|
Stability of APIs is integral as it provides the desirable effects of formulation. These can degrade with time due to hydrolysis, oxidation or microbial growth. |
| Stability of excipients
Picture Courtesy: Science Direct |
Excipients including preservatives, emulsifying agents etc. have great influence on shelf life. |
| Packaging
Picture Courtesy: Science Direct |
Suitable packaging containers that can shield formulation from exposure to air, light etc. helps in maintaining shelf life. |
| Storage conditions
Picture Courtesy: NC |
Storage conditions must be optimum to avoid any degradation caused by exposure to light, temperature and humidity. Suitable temperature for storage can be 15-30 C. |
| Microbial contamination
Picture courtesy: SlideShare |
Water-based formulations are sensitive to growth of microbes, so integration of preservatives becomes necessary. |
Conclusion:
Manufacturing of semi-solid drug products demands attention towards regulatory compliance, established process and quality control. Right choice of mixing and filling equipment ensures stable and durable drug product. Potential developments can be made by initiating technological advancements and sustainability in it, which will result in highly efficient outcome with low expenditure, so as a buyer this manufacturing provides you great opportunity to make a successful investment. For any query, you can contact AIPAK any time.
Don't forget to share this post!
Tube Filling Machine Related Posts
Tube Filling Machine Related Products
Tube Filling Machine Related Videos
CONTACT US
Tell us your raw material and project budget to get quotations within 24 hours.
WhatsApp Us: +86 181 6426 8586
Want the best price & newest pharmaceutical machinery buying guide,tips and trends sent straightly to your box?Sign up for AIPAK’s monthly newsletter,we’re free for your consultation and Offer you the most suitable solutions!
The Buyer's Guide
- Capsule Filling Buyer's Guide
- Blister Packaging Buyer's Guide
- Tablet Counting Buyer's Guide
- Tube Filling Buyer's Guide
- Cartoning Buyer's Guide
- Gummy Making Buyer's Guide
- CO2 Extraction Buyer's Guide
- Empty Capsules Buyer's Guide
- Suppository Filling Buyer's Guide
- Tablet Coating Buyer's Guide
- Tablet Press Buyer's Guide
- Softgel Encapsulation Buyer's Guide
Most Popular
- 7 Importance Of Pharmaceutical Packaging In Different Applications You Must Know
- 6 Advantages You Must Know About Tablet Counting Machine
- 8 Advantages of Blister Packaging You Must Know
- 6 Critical Applications of Automatic Capsule Filling Machine
- 6 Stations You must Know to Improve the Filling Quality of Automatic Capsule Filling Machine