Gummy Bear Osmosis Experiment
Looking for a fun gummy bear science experiment? Everyone loves eating gummy bears, apart from the scrumptious taste did you know it is a unique model for osmosis? Let’s use gummy bears for a unique science and learn how they respond to water. Gummy Bear Osmosis Experiment is such fun yet simple and this is the best way to make you understand the basic concept of osmosis that appears dull to you. Read this article; learn how to do it & try today!
1.What is osmosis?
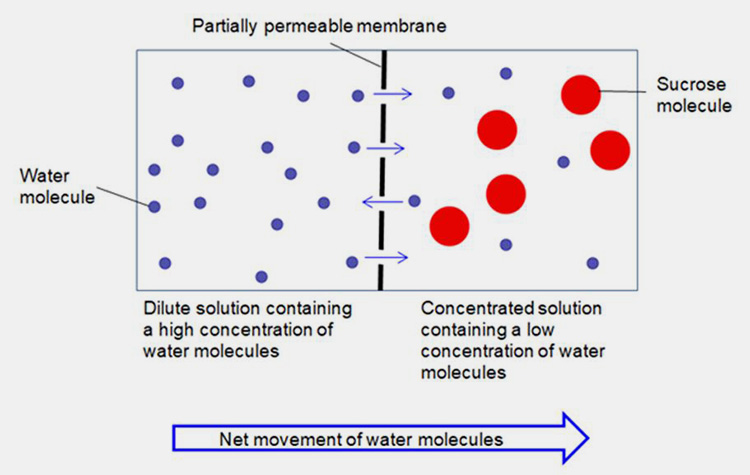
Osmosis is a special type of diffusion specific to the movement of water molecules. When there is an uneven amount of water across the semi-permeable membrane, the water molecules flow from the regions of higher water concentration to areas having a low concentration of water. This happens to even out the concentration of water and for balancing the concentration of water on both sides of a partially permeable membrane.
2.Why A Gummy Bear?
Gummy bears are attractive chewy candies, loved equally by adults and children. These little gummy confectionaries are a liquid mixture of gelatin, flavour, colour and sugar in water.
The warm gummy mixture is poured into bear-shaped moulds and on cooling, some water molecules evaporate resulting in hard gummy candies.
Gummies are like little “bubbles” having water molecules trapped inside them. The gummies behave similarly to semi-permeable membranes facilitating the transport of small molecules
3.What’s Unique About The Gummy Bear Osmosis Experiment?
Gummy bears are distinct from other sugary candies. Every other toffee dissolves in water but gummies are exclusive in this nature since they do not disintegrate in water. A gummy bear has a thin delicate gelatinous membrane that only permits water molecules to diffuse across but limits the larger one. In this way, only water molecule enters into gummy bears results in an enlargement. The gelatin and concentrated sugary mixture aid in studying the science behind osmosis. Therefore, it is an ideal candidate for the osmosis experiment.
4.What’s The Science Behind The Gummy Bear Osmosis Experiment?
These little candies blow like a balloon since they soak up water like a sponge. During the course of experiment, the gummies gradually absorb water and swell in size.
The gummy candies are typically a strong solution of sugar and gelatin with a little amount of water. The outside solution has a high concentration of water thus water flows from the outside to the inside of gummies.
The gummies have little gaps in their surface so water molecules gradually move inside this gelatin bubble but gelatin does not let sugar molecules or salt molecules inside. This osmosis process occurs to balance the amount of water exterior and interior of gummies. The gummy bear shrinks in salt solution as the gummies have more water when compared with salt solution, so molecules of water go across the gummies to the surrounding salt solution.
5.What Materials Are Required To Perform The Gummy Bear Osmosis Experiment?
Some materials used to perform the gummy bear osmosis experiment are:
- A pack of gummy bears
- Ruler
- Three bowls/glasses containing an equal amount of water
- 1 tablespoon of sugar
- 1 tablespoon of salt
- Calculator
- Timer
- Napkins
- Measuring scale
6.What’s The Procedure To Do The Gummy Bear Osmosis Experiment?
Some easy steps for conducting a gummy bear osmosis experiment include:
- Take three bowls of water and label them as plain water, sugar water, and salt water.
- Now add one tablespoon of sugar in a bowl categorized as sugar water and put 1 tablespoon of salt in a bowl labelled salt water.
- Measure the dimension (height and width) and weight of gummy bears. Record this information in a gummy bear data table.
- Place one gummy bear in each bowl.
- Wait for half a day i-e 12 hours. Then take out gummies from their bowls. Measure their size and weight and write out this data.
- Again immerse gummy bears in their particular bowls.
- The record measurement of gummies after 1 day.
- Post 2 days (48 hours), again take measurement of immersed gummies.
- What did you discover?
- Water: Gummy bear has grown bigger
- Sugar: gummy bear has grown big but less than plain water
- Salt:size has grown larger but lesser as compared to water and water +sugar.
7.Why Did Gummy Bears Get So Big?
Putting any confectionery product in water whether candies, biscuits, chocolates, cakes could results in dissolution, some materials get dissolves quickly while some takes time. Gummy bears are execeptional! They allows water molecules inside it but resists to burst or dissolved. Isn’t it amazing!
When you drop gummy bear in water you will explore it is growing big and bigger, because water molecules outside the membrane goes inside gummy that’s trapped by gelatin pockets. There are many ingredients inside the gummy bear like sugar, syrup, flavorants that try to moves outside and let the water to enters inside.
When you drop gummy bear in water+sugar solution, water flows into the bear but sugar molecules are restricted. There’s flow of water inside but not outside. This is because there must be high concentration of sugar inside as compared to outside.
Salt molecules are smaller than sugar, so they are easily dissolved in water than sugar. This mean water has salt high concentration medium outside than inside, so water moves outside the bear along with gummy content.
8.How To Calculate Weight Gain?
For calculating the weight gain of gummies, first, weigh the gummies with a measuring scale before inserting them into water.
Next measure the weight of gummies after 12 hours (or 24 hours). The gummies weigh more once they are placed in water as these have more water.
The weight gain is calculated simply by subtracting the weight of gummies that are placed in water for 12 hours (or 24 hours) from the original weight of the gummies.
Weight gain=Weight of gummies immersed for 12 hours- the original weight of gummies
9.What Happened If You Soak The Gummy Bear For 24 Hours?
The gummies will expand in length as well as in width when they are soaked in water for 24 hours. The sizes of gummies will be doubled and there will be a major increase in the width of gummies after 24 hours.
The gummies will lose their chewy jelly-like constituency resembling more like a soft gelatinous mass. Furthermore, they will have a translucent appearance as most of the colouring dye will dissolve in water and be less sweet in taste due to the addition of water.
CONCLUSION
All gummy bears grow in size because of osmosis. This is the ability of water molecules to penetrate by seeping through a semi-permeable membrane. We hope you’ve understood the concept of Gummy Bear Osmosis Experiment!
Wants to know more about gummy bears, Right? Well, we have unlimited publications on our website from manufacturing to packaging, from sweet treats to medicated ones. If you’ve any queries, contact us by sending a short message; our Hi-Tech experts are welcome to serve you! Happy Experimenting.
Don't forget to share this post!
Gummy Making Machine Related Posts
Gummy Making Machine Related Products
Gummy Making Machine Related Videos
CONTACT US
Tell us your raw material and project budget to get quotations within 24 hours.
WhatsApp Us: +86 181 6426 8586

Want the best price & newest pharmaceutical machinery buying guide,tips and trends sent straightly to your box?Sign up for Aipak’s monthly newsletter,we’re free for your consultation and Offer you the most suitable solutions!
The Buyer's Guide
- Capsule Filling Buyer's Guide
- Blister Packaging Buyer's Guide
- Tablet Counting Buyer's Guide
- Tube Filling Buyer's Guide
- Cartoning Buyer's Guide
- Gummy Making Buyer's Guide
- CO2 Extraction Buyer's Guide
- Empty Capsules Buyer's Guide
- Suppository Filling Buyer's Guide
- Tablet Coating Buyer's Guide
- Tablet Press Buyer's Guide
- Softgel Encapsulation Buyer's Guide