Aseptic Processing and Packaging in Pharmaceutical Industry
Have you ever thought about how life-saving drugs stay sterile throughout the supply chain? How do pharmaceutical manufacturers ensure their products remain free of microbes and other contaminants? How medications are delivered in their purest form to patients?
The answers to all these questions are grounded in a life-changing concept “Aseptic Processing and Packaging in Pharmaceutical Industry”. Can you imagine working in a facility where a minute dust smidgeon, a drifting particle, or a single bacteria can risk the safety of a substantial stockpile of treatment agents?
Certainly not, because pharmaceutical manufacturing makes use of aseptic processing and packaging to produce and package in an aseptic environment that averts all possible chances of contamination. Curiosity piqued? Let's explore the fascinating realm of aseptic processing together.
1.What is aseptic processing and packaging in the pharmaceutical industry?

Aseptic Processing and Packaging in Pharmaceutical Industry
It is the term that defines a manufacturing process in which sterile containers, such as vials, ampoules, and bags are loaded with sterile pharmaceutical and biopharmaceutical products (liquids and lyophilized powders) under aseptic conditions. In aseptic processing and packaging, the products do not undergo terminal sterilization. So, it is useful for thermos-sensitive medications, such as biologics, biosimilars, vaccines, and other parenteral drugs.
Different steps, for instance, sterilization of equipment, materials, aseptic filling and sealing, and packaging are carried out in this approach. Moreover, it takes place in a specialized environment (cleanroom) to prevent the access of microbes or other physical impurities to sterile products.
2.How to pharmaceutical industry benefit from aseptic processing and packaging?
Aseptic processing and packaging in the pharmaceutical industry have the highest beneficial power because of their potential to keep medication efficacious and potent. It maintains the peak performance of various treatments and perverse their integrity. Now, let’s look into the remarkable benefits of aseptic processing and packaging for the pharmaceutical industry:
A Barrier Against Contamination
A Barrier Against Contamination
Aseptic processing and packaging are mostly employed in the industrial sector because it is perfect for creating a sterile environment and ultimately safeguarding the medications from pathogens, pyrogens, particular substances, dust, fragment residues, etc. Thus, it is effective in maintaining the health of individuals and prevents contamination-related adverse reactions in the body upon medication administration.
Ideal for Quality Assurance
Ideal for Quality Assurance- Picture Courtesy: Cytiva
By employing strict quality control techniques throughout the aseptic processing and packaging in the pharmaceutical industry, you can attain the ultimate standard of medication quality. From formulation preparation to final delivery, there is no compromise on medication purity. Furthermore, rigorous sterility testing is carried out at every step of aseptic processing and packaging to remove impure preparations from moving further into the production line.
Processing Thermo-Sensitive Drugs
Best for Processing Thermo-Sensitive Drugs- Picture Courtesy: Salas O’Brien
Different types of biopharmaceutical drugs, such as biologics, hormones, or vaccines are denatured upon contact with high temperatures. So, how are they packed? Aseptic processing and packaging are fitting for filling and packaging these products, as it does not require post-packaging sterilization. It preserves the biological activity, structural composition, and efficacy of these complex biological formulations.
Prolongs Shelf-life
Prolongs Shelf-life- Picture Courtesy: Svaya Robotics
Aseptic processing and packaging make use of aseptic packaging materials, such as glass or plastic that have peak barrier characteristics and are ideal for protecting delicate medications from harsh environmental conditions of oxygen, moisture, and light. These agents may degrade the structural bonding in medications. However, high-barrier materials are excellent for stabilizing products and extending their usable period.
Meeting Regulatory Standards
Meeting Regulatory Standards- Picture Courtesy: Parker Hannifin
Regulatory adherence is the cornerstone of aseptic processing and packaging in the pharmaceutical industry. It is instigated in pharmaceutical facilities to conform to strict regulatory rules issued by global drug and health agencies, such as the FDA, EMA, and WHO. With this approach, you can easily decrease the likelihood of product rejection and recalls as well as monetary penalties.
Reduces Wastage and Production Costs
Reduces Wastage- Picture Courtesy: Contract Pharma
By implementing practices of aseptic processing and packaging, you can circumvent the issues of contamination, which eventually leads to less product wastage during production. Consequently, you can cut down your manufacturing expenses and acquire more financial stability.
3.What are the common industries that need aseptic processing and packaging?
Are you speculating about applications of aseptic processing and packaging? You’ll certainly find it in industries that require absolute sterility and purity. Sensitive products and individual therapies must be processed using aseptic processing and packaging. Some common industries that employ aseptic processing and packaging include:
Pharmaceutical Industry
Aseptic Processing and Packaging in Pharmaceutical Industry- Picture Courtesy: Woodstock Sterile Solutions
It is the core industry in which aseptic processing and packaging has forged its path. Pharmaceutical manufacturers and developers regularly utilize this technique to keep their injectable, parenteral drugs, vaccines, nasal sprays, ophthalmic solutions, IV fluid, lyophilized powders, ear drops, and more, safe and sterile. With it, you can guarantee product stability to your customers and healthcare sectors.
Biotechnology Industry
Biotechnology Industry- Picture Courtesy: News-Medical
In the biotechnology industry, various kinds of biopharmaceuticals, cell products, gene therapies, cell cultures, monoclonal antibodies, recombinant proteins, and clogging factors are processed. They are delicate products, requiring the highest levels of sterility because impurities disrupt their structural pattern and inhibit their functionality. Therefore, aseptic processing and packaging have a central job in this sector to stop contaminants from interfering with the structural and physical characteristics of these products.
Medical Care Industry
Medical Care Industry- Picture Courtesy: Medical Product Outsourcing
What part does aseptic processing and packaging have in the medical sector? In this industry, various types of medical instruments, for instance, catheters, syringes, IV tubes, surgical instruments, and implantable tools are housed in aseptic containers through aseptic processing and packaging techniques. The human body is directly exposed to these devices, so they must be sterile.
Food and Beverage Industry
Aseptic Processing in the Food Industry- Picture Courtesy: Packaging Getaway
At first, it may seem odd but aseptic processing and packaging are indispensable for the food industry. Why? Because it holds the responsibility of prolonging the consumable life and expiry period of sensitive food and beverages, such as milk, yogurt, juice, sauce, baby food, pastes, and others. There is no need to add preservatives to these products, as this sterile technique is perfect for preventing microbial colonization and keeps food from getting spoiled.
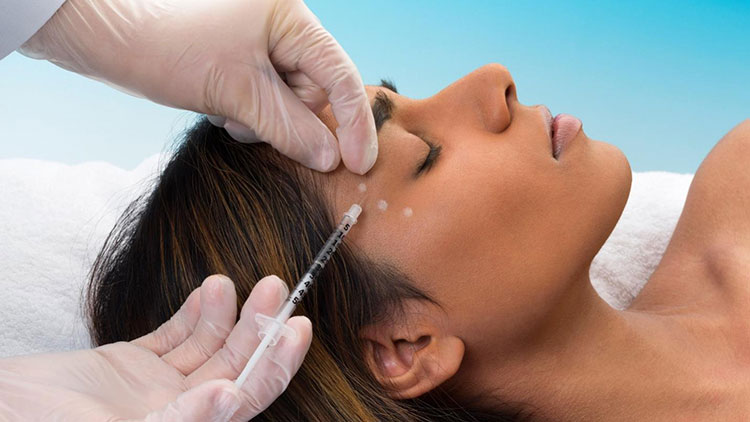
Cosmetic Industry
Botox Injections in the Cosmetic Industry- Picture Courtesy: Allure
Nowadays, more and more customers are demanding hygienically sound cosmetic products because of rising health awareness. Thus, aseptic processing and packaging is an excellent addition to the cosmetic industry and serves the function of safeguarding the sterility of precarious cosmetic and dermatological products, for example, skincare creams, lotions, medical-grade cosmetics, Botox, and eye drops.
Diagnostic Industry
Diagnostic Industry
Diagnostic kits, reagents, and other sampling devices must be packed through aseptic processing and packaging because even a minute amount of contaminant can negatively impact the accuracy and precision of diagnostic tests. Inaccurate tests result in false positive or false negative outcomes, leading to errors in treatment approaches.
4.What are the steps and techniques of aseptic processing and packaging?
A seamless coupling of biological, physical, and chemical techniques is utilized in aseptic processing and packaging to keep products from getting contaminated during operational setup. The growing focus on health and wellness is driving the demands of aseptic techniques. Let’s discover multifaceted steps and techniques of aseptic processing and packaging in the pharmaceutical industry:
Product Sterilization
Product Sterilization- Picture Courtesy: Getinge
In this step, the products are first sterilized to eradicate various types of impurities and biological pathogens. Some product sterilization approaches are:
| Steam Sterilization | It is also called autoclaving and is typically utilized for processing heat-tolerant products. High temperature and pressure steam is passed through products to kill different microbes, their spores, and endotoxins. It penetrates the materials and exchange heat energy to deactivate microbial proteins. |
| Filtration | It is used in place of steam sterilization for heat-sensitive liquids, such as biologics. It utilizes ultrafine filter membranes to separate microbes from products. |
| Radiations | As the name suggests, in this method, high-energy radiations, for instance, gamma, ultraviolet, or electron beams penetrate products to sterilize them. It destroys the physical structure of vital molecules in bacteria, viruses, and fungi. |
| Dry Heat Sterilization | It is ideal for sterilizing moisture-sensitive products. High temperatures are employed for destroying microorganisms. In this approach, oxidation terminates microbial cells. |
Equipment and Packaging Sterilization
Equipment and Packaging Sterilization- Picture Courtesy: Medical Product Outsourcing
After product sterilization, it is now the turn of equipment and containers to undergo sterilization. This step is executed to remove different kinds of biological agents, spores, and by-products of microbes from filling and packaging equipment in the pharmaceutical industry.
| Sterilization-in-Place (SIP) | Many advanced pharmaceutical production lines are equipped with this system. It is automated and saves time in equipment sterilization. It employs steam or strong chemical agents that kill microbes from both the interior and exterior of instruments without disassembling their components. |
| Chemical Sterilization | For detoxifying diverse types of devices and containers in pharmaceutical facilities, strong chemical agents, for example, hydrogen peroxide or ethylene oxide are employed. It sterilizes surfaces and inner parts of equipment by spraying chemicals on them. |
| Depyrogenation Tunnel Sterilization | In this sterilization technique, high temperatures (typically around 250°C) are used for specific periods to inhibit pyrogens and other microbes. Afterward, the containers are cooled slowly to avert thermal shock and cracking. |
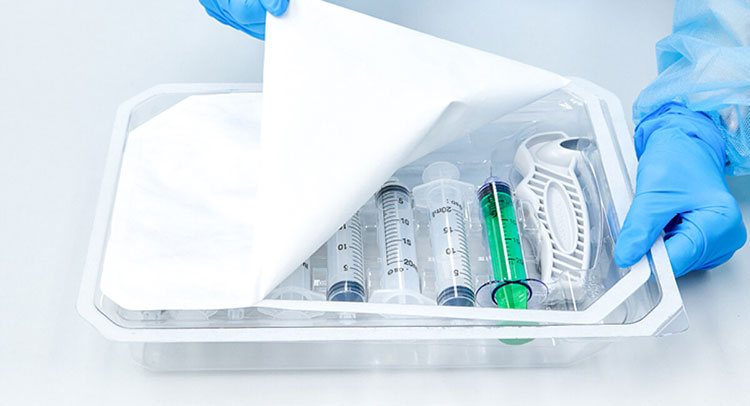
Aseptic Filling and Sealing
Aseptic Filling and Sealing- Picture Courtesy: Packaging Strategies
Usually, after equipment sterilization, the pharmaceutical drugs and biologics are loaded in different types of sterile containers, such as vials, ampoules, syringes, bags, etc using robotic filling systems and other isolators. These systems act as a physical barricade that reduces the interaction of humans and equipment, thereby, preventing microbes from gaining access to filling systems. After filling, containers are fully enclosed with durable closures, such as rubber stoppers instantly to prevent contamination.
Validation and Testing
Validation and Testing
How quality of the product is validated? Finally, the sterility and quality of products and their packaging are checked and validated through cell culture testing, vacuum decay testing, and dye ingress testing. It investigates microbial contamination. Also, the sealing of different containers is analyzed to ensure their integrity.
5.What equipment is typically used for aseptic processing and packaging?
What kind of equipment should be employed for aseptic processing and packaging? This question is often posed by pharmaceutical manufacturers. There are some specialized instruments for aseptic processing and packaging in the pharmaceutical industry. Some useful devices are mentioned below for your ease:
Filtration Systems
Filtration Systems- Picture Courtesy: EMD Millipore
They are integral systems in the aseptic processing and packaging for upholding purity requirements. Different kinds of filters and molecular weight cutoff membranes with small pore sizes are utilized for extracting the tiniest of molecules, such as endotoxins, pyrogens, and, pathogens.
Ultrasonic Washing Machine
AIPAK Ultrasonic Washing Machine
Ultrasonic washing machine is the latest device in a line of cleaning equipment and incorporates cutting-edge technology i-e high-frequency ultrasonic or sound waves around 20kHz-400-kHz to decontaminate and wash diverse pharmaceutical containers, for instance, vials, ampoules, syringes, or bottles. It cleans the smallest of corners and pockets and dislodges debris, biofilms, spores, and, other fragments.
Depyrogenation Tunnels
AIPAK Depyrogenation Tunnels
As the name indicates, this unit is effective for eradicating harmful pyrogens or endotoxins through dry heat. Pharmaceutical containers are subjected to heated temperatures for particular intervals to attain precise sterilization and pyrogen denaturation. This device neutralizes endotoxins by degrading their biological components.
Aseptic Filling and Sealing Machines
AIPAK ENGINEERING Vial Production Line
They are the principal component in the aseptic processing and packaging for sterile loading and sealing of a variety of medications, such as chemotherapy, hormones, parenteral drugs, and others in sterile containers and avert the ingress of contaminants. Ultra-precise pumps regulate the flow rate of products in these systems and can accommodate a wide array of viscous medications.
IV Bag Filling and Sealing Machine
AIPAK ENGINEERING IV Bag Filling and Sealing Machine
It is a dedicated unit to load and seal intravenous bags under aseptic conditions. In this device, the latest dosing systems, for instance, peristaltic pumps or flow meters quickly and precisely fill IV solutions, total parenteral nutrition, blood plasma, etc in PVC or non-PVC bags. Precise heat or ultrasonic sealing devices are applied to create leak-proof seals on IV bags.
Isolators
Isolators- Picture Courtesy: Syntegon
It is a type of containment device and is increasingly utilized in the pharma sector to maintain an enclosed and sterile environment for steps, such as filling, sealing, quality testing, and managing perilous chemicals. It is vital in shielding both medication and personnel from poisonous substances and contaminants.
Restricted Access Barrier Systems (RABS)
RABS- Picture Courtesy: Telstar
It is an up-to-date kind of containment system, specially developed to boost sterility in pharmaceutical units. It offers a physical enclosure to isolate the sterile operational setup and operators to avert contamination chances and maintain stringent aseptic conditions. However, it allows human intervention for maintenance with glove ports.
6.What are the classes of cleanroom environments? How do they relate to aseptic processing and packaging?
A cleanroom environment is the mainstay of aseptic processing and packaging and is exploited with the sole purpose of decreasing the incidence of airborne particles and other harmful toxins inside the aseptic processing and packaging area. You can routinely see them in pharmaceutical, biotechnology, electronic, and medical care settings.
There are four classes of cleanrooms depending on the quantities of airborne impurities permitted in a particular amount of air. Cleanroom criteria are specified by two standards that are:
- ISO 14644-1 (International Standards Organization)
- US Federal Standard 209E (used frequently in the US)
These standards measure particle sizes in micrometers. Let’s discuss categories of cleanrooms and their importance in aseptic processing and packaging in the pharmaceutical industry:
Grade A Cleanroom
AIPAK Engineering Grade A Cleanroom Solutions
It is also referred to as ISO class 5, class 100 (US FED STD 209E), or critical zone. This class has the most strict criteria for cleanliness, as aseptic processing activities, for instance, vial filling, ampoule loading, product dispensing, capping, or sealing take place in this type of cleanroom. Typically, RABS or laminar airflow units make up ISO class 5. According to ISO, it should not have more than 100 particles per cubic meter. Positive air pressure is kept in a grade A clean room to prevent external contamination.
Grade B Cleanroom
AIPAK Engineering Cleanroom Solutions
It is termed as iso class 7 or class 10,000 cleanroom and normally consists of a surrounding room where aseptic processing is happening under laminar airflow. It is a backdrop area for a grade-A clean room. It is maintained in pharma and other research laboratories.
Grade C Cleanroom
AIPAK Engineering Grade C Cleanroom Solutions
It is iso class 8 or class 100,000 for non-vital production zones. In this cleanroom, mostly material preparation or component compilation is performed. Containers are washed and processed in these areas before sterilization.
Grade D Cleanroom
AIPAK Engineering Cleanroom Solutions
It is an ISO class 9 cleanroom, comprising support areas, and has minimal particulate and microbial contamination for cleanrooms. Raw materials are received and placed in the grade D cleanroom.
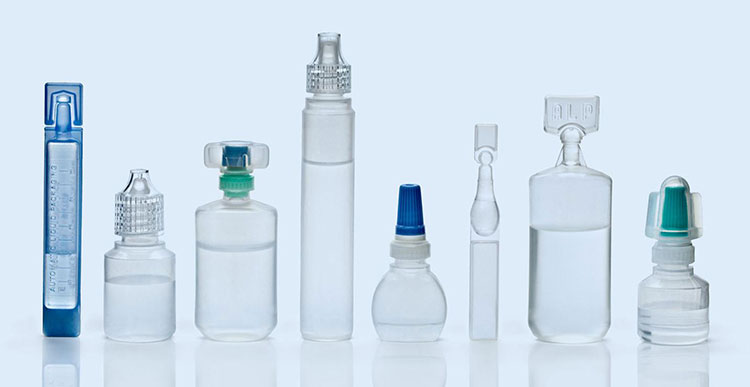
7.What packaging containers are best for aseptic processing and packaging?
Various kinds of packaging containers are used in aseptic processing and packaging in the pharmaceutical industry to pack containers that are excellent in retaining the sterility and potency of healthcare products. Some of the containers include:
Glass Containers
Glass Containers
They are clear for visual check for contents and chemically non-reactive, hence, apt for the majority of pharmaceutical medications. Furthermore, they are heat-tolerant and are easily heat-treated. They further include:
| Vials | They are cylindrical containers, available in a variety of sizes, and are appropriate for packing diverse types of parenteral and lyophilized powders. |
| Ampoules | They are glass containers that are melted and fused to create a permanent closure and are broken for accessing drugs. They store single doses of drugs. |
| Cartridges | They are pen-like injectors and are exercised for housing insulin. |
Plastic Containers
Plastic Containers- Picture Courtesy: BD
They are flexible, lightweight, and non-breakable and are best known for their superb molding potential and pliability. They are also inert to certain chemicals and impermeable to water. There are diverse kinds of plastics, for example, polypropylene, polyethylene, and, polyethylene terephthalate.
| IV Bags | They are made from PVC and non-PVC polymers and are easily collapsible. They are lightweight and do not allow air entry. Specialized aseptic filling and sealing machines are utilized for filling IV bags, such as non-PVC soft bag IV solution production lines. They house parenteral solution, nutrition therapy, and, electrolyte fluids. |
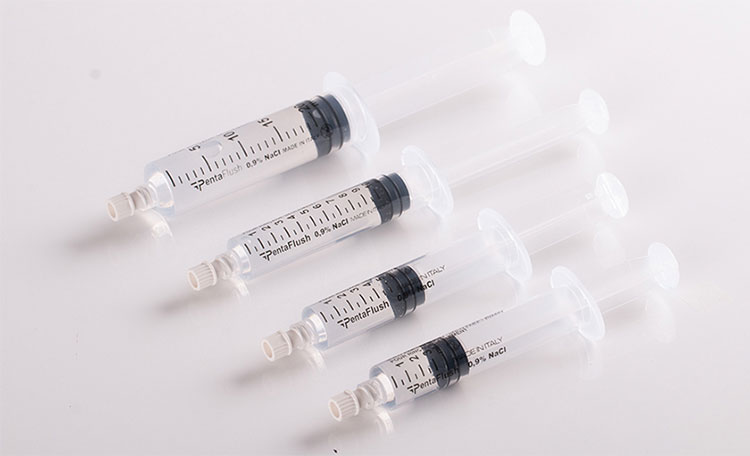
Pre-filled Syringes
Pre-filled Syringes- Picture Courtesy: Delta Med
It is filled in a sterile and aseptic environment; consequently, has zero chance of contamination. Moreover, using these syringes, you can have accurate dosing and lower preparation time. It is prepared from plastic, glass, and, silicon to assist in smooth plunger flow.
Blister Packs
Blister Packs
They are appropriate for enclosing single doses of tablets and capsules and prevent their destruction by environmental agents. They are easy to utilize and sustain the purity of products till the opening of packs. It is manufactured using plastic films and aluminum foils.
Flexible Pouches
Flexible Pouches- Picture Courtesy: Eagle Flexible Packaging
They are created employing a combination of high-barrier multi-layer polymers to keep contents safe from interference by moisture, air, and light. You can customize them in any shape or size. In the pharmaceutical sector, liquids, suspensions, creams, and, gels are loaded in flexible pouches.
8.What are the differences between aseptic and non-aseptic processing and packaging?
Aseptic Processing and Packaging- Picture Courtesy: Parker Hannifin
Two distinct processing and packaging approaches- aseptic and non-aseptic processing and packaging- are employed in the pharmaceutical and food industry. They vastly differ from each other, based on sterilization and product handling. The detailed comparison between both these approaches is detailed below:
| Feature | Aseptic Processing and Packaging | Non-aseptic Processing and Packaging |
| Definition | It entails sterilization of the product as well as its container separately. Afterward, filling and sealing are carried out in sterile areas. | It does not require the sterilization of materials and products before filling. The packed contents can be sterilized up to some extent post-packaging but it usually depends on preservatives for stability. |
| Sterilization Standards | The contents, packaging, and closures must be sterilized and the product must be filled and sealed in a Grade A cleanroom. | There are no specific guidelines for sterile conditions, however, contamination is managed by implementing protocols of hygiene and cleanliness. |
| Environment | It is carried out in cleanrooms and incorporates HEPA filters to retain low particulate levels in the surrounding air. | It is performed in typical manufacturing areas with less stringent contamination control standards. |
| Application | It is used for products, where sterility is a top requirement. It packs injection solutions, eye drops, biologics, vaccines, hormones, and other biopharmaceuticals. | It is mostly utilized for housing tablets, capsules, syrups, creams, and ointments. |
| Equipment | It exploits specialized devices, for instance, isolators, RABS, filtration systems, and aseptic filling machines. | It utilizes standard pharma processing devices, like tablet presses, capsule fillers, mixers, blenders, etc. |
9.What are the common risks associated with aseptic processing and packaging?
Utmost care is taken to minimize risks associated with aseptic processing and packaging in the pharmaceutical industry by implementing rigorous sterility measures. Still, occasionally you’ve some risks that can jeopardize the purity and potency of pharmaceutical products. But with proactive measures, you can easily mitigate them. Let’s discuss some common risk and their resolution approaches.
Contamination Risk
Contamination Risk
Did you encounter contamination in your facility? Two types of contamination are regularly observed in the industries- microbial and particulate contamination. Human personnel, instrument damage or uncleanliness, raw materials, and packaging films are sources of contamination. It can result in product recall, regulatory penalties, faults in pure products, and threaten the health of patients.
Solutions
There are a number of ways to resolve this challenge, such as installing HEPA-filtered laminar hoods in cleanrooms. Implementation of stringent hygienic protocols, like the use of protective clothing, gloves, etc. Adhering to routine environmental examination schedule of air, surfaces, and working operators. Prioritizing the use of pre-sterilized materials and sterilized devices and medications.
Environmental Risk
Environmental Risk
They are related to cleanroom breaches and inaccurate environmental settings. Sometimes, frequent opening of doors in cleanrooms, irregularities in air flow, or poor positive pressure can result in cleanroom infringements. Also, defects in air handling devices or their improper maintenance are associated with poor environmental regulation. These shortcomings allow the entry of different types of microbes or particles into products.
Solutions
You can easily address this issue by keeping cleanroom zones at differential pressure to keep impurities from entering cleanrooms. It is advised to utilize airlocks on doors and reduce operator flow in clean zones. Follow proper maintenance schedule for air handling systems. Also, you should routinely perform cleanroom qualification.
Packaging Failures
Packaging Failures- Picture Courtesy: Amcor
This issue often arises in aseptic processing and packaging. There are numerous reasons for this problem, for instance, incorrect sealing of pharmaceutical containers, wear and tear of packaging vessels, degradation of packaging barriers, etc. It is a major factor in the uncleanliness of products.
Solutions
You can solve this problem by performing container-closure integrity testing, for instance, vacuum decay or dye testing. Secondly, it is recommended to use packaging materials that are pre-sterilized. You should regularly validate and calibrate sealing machines for correct operation. Also, prevent packaging defects by properly storing and handling packed products.
Insufficient Sterilization
Insufficient Sterilization- Picture Courtesy: Syntegon
Yes, you could also face problems of insufficient aseptic processing and packaging of pharmaceutical medication or devices. What are its causes? Low sterilization time, temperature, and fewer chemical exposures are core reasons attributed to this problem. It could have a severe impact, for instance, ingress of endotoxins, bacterial, viral, or fungal pathogens.
Solutions
Although it is a serious risk, however, it is effortlessly managed by verifying sterilization procedures using biological indicators and media culture. Experts suggest employing numerous sterilization approaches, for example, steam, chemicals depending upon the product's nature. Also, make sure to check sterilization settings regularly. Moreover, you should revise sterilization SOPs to adhere to the latest best sterilization practices.
10.What are the up-to-date technological developments in aseptic processing and packaging?
Every industrial technology is evolving at a much faster pace and aseptic processing and packaging is no exception to rule. These technological advancements are a must for improving sterility facets as well as for regulatory compliance in pharmaceutical sectors. Some of the latest advancements in aseptic processing and packaging are discussed below:
Robotics and Automation
Robotics and Automation- Picture Courtesy: Parenteral Drug Association
Engineers are pioneering the development of advanced robotics devices (collaborative robots) for industries with aseptic processing and packaging to cut down the communication between equipment or products and humans. This is ideal for increasing consistency, batch reproducibility, accelerated production fluidity, and lower contamination.
Single-Use Technologies (SUTs)
Single-Use Technologies (SUTs)- Picture Courtesy: Single Use Support
Also, pharmaceutical manufacturers are employing disposable membranes, filters, and pipes for aseptic processing and processing. The use of pre-sterilized single-use systems is becoming a standard routine for aseptic processing. It is excellent for minimizing the cleaning and sterilization needs and facilitates quick changeover.
Real-Time Investigation
Real-Time Investigation
Nowadays, aseptic processing and packaging are aided by the application of real-time monitoring IoT sensors. You can utilize these latest data analytics units to perform predictive maintenance and sterility validation. It instantly identifies errors in a cleanroom environment and allows better contamination control.
Sustainable Packaging
Sustainable Packaging- Picture Courtesy: Meyers Printing
Increasing awareness of environmental needs has encouraged manufacturers to employ sustainable packaging in aseptic processing and packaging. With the utility of biodegradable, recyclable, lightweight, and sterilized plastic and innovative materials, you can contribute to lowering the environmental impact and satisfy consumer demand for green enclosures.
Conclusion
Aseptic processing and packaging are considered a magician in the pharmaceutical industry that guarantees the absence of microorganisms and other impurities in final products. No pharmaceutical industry functions correctly without this technology. Moreover, patient treatments are impaired without aseptic processing and packaging. It is not just a manufacturing process but also a salvation. With the help of this approach, you can ensure that medications are delivered to patients in potent and pure form. Need aseptic processing solutions? AIPAK is here to help.
Don't forget to share this post!
Blister Packaging Machine Related Posts
Blister Packaging Machine Related Products
Blister Packaging Machine Related Videos
CONTACT US
Tell us your raw material and project budget to get quotations within 24 hours.
WhatsApp Us: +86 181 6426 8586
Want the best price & newest pharmaceutical machinery buying guide,tips and trends sent straightly to your box?Sign up for AIPAK’s monthly newsletter,we’re free for your consultation and Offer you the most suitable solutions!
The Buyer's Guide
- Capsule Filling Buyer's Guide
- Blister Packaging Buyer's Guide
- Tablet Counting Buyer's Guide
- Tube Filling Buyer's Guide
- Cartoning Buyer's Guide
- Gummy Making Buyer's Guide
- CO2 Extraction Buyer's Guide
- Empty Capsules Buyer's Guide
- Suppository Filling Buyer's Guide
- Tablet Coating Buyer's Guide
- Tablet Press Buyer's Guide
- Softgel Encapsulation Buyer's Guide
Most Popular
- 7 Importance Of Pharmaceutical Packaging In Different Applications You Must Know
- 6 Advantages You Must Know About Tablet Counting Machine
- 8 Advantages of Blister Packaging You Must Know
- 6 Critical Applications of Automatic Capsule Filling Machine
- 6 Stations You must Know to Improve the Filling Quality of Automatic Capsule Filling Machine