What Is the Pharmaceutical Process Technology of Solid Dosage Form?
Solid dosage form is one of the three forms of drugs, and its quality directly affects people’s physical health. In order to improve the quality of solid dosage forms, it is necessary to continuously improve the pharmaceutical process technology of solid dosage form and solve various problems.
Therefore, the post analyzes the application value, common processes, and pharmaceutical process improvement measures of solid dosage forms, hoping to provide support for further improving the quality of solid dosage form pharmaceuticals.
1.What Is A Solid Dosage Form?
Solid Dosage Form - Sourced: Crystal Pharmatech
Solid dosage forms (solid formulations) are a general term for dosage forms that exist in a solid state. Their common absorption pathway is mainly to orally administer solid preparations, dissolve them first after oral administration, and then absorb them into the bloodstream through the gastrointestinal mucosa, and further exert their pharmacological effects.
Compared with liquid dosage forms, the solid one has good physical and chemical stability, lower production costs, convenient packaging and transportation, and is easy to take and carry.
2.What Are the Types of Solid Dosage Forms?
The commonly used solid dosage forms in the pharmaceutical field include granules, tablets, capsules, and other diverse types. Most patients are willing to receive solid drug treatment and have excellent patient compliance. I will introduce four types of solid dosage forms in detail.
| Types of Solid Dosage Forms | Description |
| Powder
Powder - Sourced: PCCA |
Dry powder preparation made by grinding and uniformly mixing drugs and suitable excipients. Can be taken orally or topically. Oral powder can be dissolved in water or dispersed in water for consumption. Topical powder can be used on the skin, mouth, throat, or cavity. |
| Granule
Granule - Sourced: KALYDECO (ivacaftor) |
Dry granular preparations with a certain particle size made by mixing raw materials and suitable excipients. It can be understood as adding adhesive on the basis of the powder to make each component powder bond into larger particles. |
| Tablet
Tablet - Sourced: Felix |
Round or irregular solid tablets made from raw materials and suitable excipients. |
| Capsule
Capsule - Sourced: Ascend Packaging Systems |
Solid dosage form made by filling raw drugs or suitable excipients into hollow capsules or sealing them in soft capsule materials. |
3.What Are the Advantages of Solid Dosage Form?
Solid dosage form is a common form of medication, why so many medicine use this types? What are the advantages of this drug?
Strong physical stability
Strong Physical Stability - Sourced: learnGxP
Compared with other forms of drug formulations, solid dosage forms have stronger physical stability, are easier to produce and store, and are less affected by external environment and packaging materials.
Low cost
The production cost of solid pharmaceuticals is relatively low, with low requirements for transportation vehicles, which reduces transportation costs and overall reduces drug costs.
Convenient for patients
Convenient for Patients - Sourced: AU Health News
It is more convenient for patients to carry solid drug dosage forms when going out. Just need to carry the required amount of medication, no need to carry the entire bottle of medication, small footprint, convenient to carry.
4.What Is the Pharmaceutical Process Technology of Solid Dosage Form?
From the previous text, you have got the 4 main types of solid preparations. Now, you will get the pharmaceutical process technology of solid preparations.
Process technology of powder
Process Technology of Powder - Sourced: The Manufacturer
Powder is a powdered medication obtained by crushing and mixing drugs and auxiliary drugs. In production, it is necessary to crush the medicinal materials, screen out inferior medicinal ingredients, add auxiliary medicinal materials to high-quality medicinal powder, stir evenly, determine the dosage of the medicine, and finally strictly inspect the dosage and composition of the medicine before packaging.
Compared with other drug formulations, powder drugs are more suitable for children to take, but their drawback is that they are prone to excessive dosage and are more affected by external environments, making them more susceptible to moisture.
Process technology of granule
Process Technology of Granule - Sourced: Tab Tech Solution
The medicinal materials and excipients need to be processed first, and the particle preparation processes that can be adopted include direct molding, wet molding, and dry molding.
Direct molding:
Based on wet molding technology, preheat and dry the chemical components, and then add wetting agents to turn them into soft chemicals, which can simultaneously apply the steps of soft material, drying, and granulation;
Wet molding:
It is necessary to mix an appropriate amount of auxiliary ingredients and special adhesives into the drug, stir evenly, add wetting agents, use drug molding equipment to extrude the drug, dry the drug, and complete the packaging. The advantage of this method is that it has a higher molding rate;
Dry molding:
In this process, the workers should add an appropriate amount of auxiliary materials to the dry paste powder, stir thoroughly, and use pressure equipment to make it into particle shape. This method requires facing many uncertain factors during use, which can reduce the molding rate of the drug.
Process technologyof tablet
Process Technology of Tablet - Sourced: Freepik
The production process of tablet drug preparations mainly involves granulating and mixing the active ingredients and excipients, and then using molding equipment to press them into tablets. Tablets are usually taken orally, mainly including regular tablets, chewable tablets, sublingual tablets, lozenges, enteric coated tablets, and sustained-release tablets.
Dry pressing:
Dry granulation pressing method, powder direct pressing method, and crystallization pressing method can be used to mix and granulate the active ingredients and excipients of the drug without contacting the liquid, ensuring that the quality ingredients are the same and have suitable flowability. Tablet press machine can be used to make them into tablets;
Wet pressing:
Mixing the active ingredients of the drug with excipients, wetting them, and drying them to make tablets. From a functional perspective, enteric coated tablets and sustained-release tablets require the use of special excipients. Through the aforementioned manufacturing methods or more complex formulation techniques such as functional coating, laser drilling, melt granulation, etc., the drugs can be released at specific locations or slowly released after entering the body, thereby achieving a sustained release effect.
Process technologyof capsule
Process Technology of Capsule - Sourced: Nutraceutical Business Review
The specific preparation techniques used for different types of capsule preparations are different, mainly including:
Soft capsules: special soft capsules are required to encapsulate the pharmaceutical ingredients;
Hard capsule: Hollow capsule material is required to add the pharmaceutical ingredients into it;
Enteric coated capsules: The capsule material is prepared using a special material of soft and hard capsules. The drug shell does not dissolve in the stomach, but dissolves in the intestine, and the released ingredients can also be beneficial to the human body.
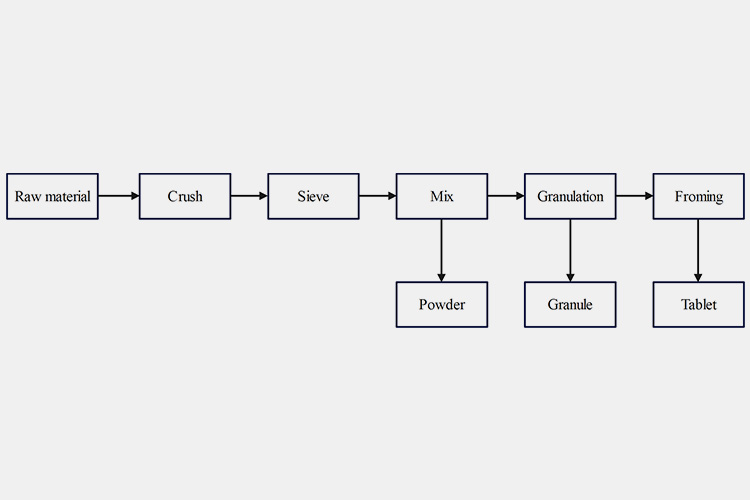
5.What Is the Manufacturing Process of Solid Dosage Form?
The process commonly used by most pharmaceutical companies in solid dosage form pharmaceuticals is shown in following figure.
Manufacturing Process of Solid Dosage Form
Preparing raw material
Selecting appropriate drug ingredients based on the desired drug efficacy, generally including active ingredients and excipients.
Crushing
Crushing is mainly the process of using mechanical force to break down large solid materials into suitable sizes. In the pharmaceutical industry, other methods can also be used to break solid drugs into micro powders.
Sieving
After the drug is crushed, the powder varies greatly in thickness. In order to meet the requirements, the process of separating the coarse powder from the fine powder is called sieving, and this mesh like tool is called a sieve or sieve.
Mixing
The main powder and excipient must be weighed separately according to the prescription. In order to ensure the quality of the tablets, you should mix several times.
The above steps are necessary for all types of solid dosage forms, and the following steps are different from types of drugs:
Directly packaging the crushed powders can obtain drug powder;
If the particles are dried, granulated, and crushed, granular can be obtained;
After compressing the particles, tablets can be obtained;
If the crushed drug particles are packaged into capsules, capsule can be obtained.
6.What Are the Common Issues of Pharmaceutical Process Technology of Solid Dosage Form?
In the production process of solid dosage forms, there may be some problems, including raw materials, production process issues, and so on.
Raw material selection issue
Raw Material Selection Issue - Sourced: Evercare Medical
Solid dosage forms require the fixation of drug raw materials, which requires the use of adhesive materials. If the adhesive effect of the pharmaceutical material itself is good, the pharmaceutical effect can be further optimized.
However, in the production of existing solid pharmaceutical preparations, some small pharmaceutical companies do not attach enough importance to adhesive materials when selecting raw materials, which affects the quality of solid preparations.
Quality issues in drug production
Compared to other forms of drugs, solid preparations require more complex preparation processes, and the quality of the drug is influenced by multiple factors. For example, poor mixing and looseness of drugs are often found in solid pharmaceutical preparations, which can have adverse effects on drug content and efficacy, affecting drug efficacy.
For example, the powder cannot flow, there is a significant difference in the loading amount during compression, the hardness is uneven, and there are issues such as stickiness and roughness. As a result, the uniformity of the prepared finished drug is poor and does not meet the specified labeling amount.
Packaging issues
Packaging Issues - Sourced: Reddit
The problem of excessive packaging of drugs can lead to a significant increase in drug prices. In the absence of significant changes in drug efficacy and rapid price growth, it will inevitably affect consumers’ purchasing decisions, which in turn will affect the sales of solid dosage forms.
Excessive packaging not only leads to resource waste, but also affects brand image. The slow degradation process of the packaging materials used poses a threat to the surrounding environment.
7.How to Solve the Common Issues of Pharmaceutical Process Technology of Solid Dosage Form?
Corresponding improvement measures are also needed to address quality control issues in the preparation of solid dosage forms. Mainly including the following points:
Scientific selection of raw materials
Scientific Selection of Raw Materials - Sourced: EPO
To ensure the quality of solid pharmaceutical products, it is necessary to choose medicinal materials with higher adhesion to provide support for improving the success rate of pharmaceutical production. To choose reasonable raw materials for medicinal herbs, it is necessary to:
1)Comprehensively analyze the disease situation treated by drugs;
2)Check the dryness and humidity of drugs to ensure proper adhesion;
3)Attention should be paid to the selection of excipients to avoid adverse reactions with the medicinal materials themselves;
4)The storage temperature and humidity of medicinal materials should be reasonable to avoid the loss of efficacy and even denaturation of medicinal materials in excessively high temperature environments;
5)The drug formula needs to be reasonably controlled. For example, the preparation of calcium alginate capsules requires different experiments to ensure that various components and temperatures in the medicinal materials reach their optimal state.
Intensifyingthe quality inspection
Intensifying the Quality Inspection - Sourced: LinkedIn
The specific types of solid dosage forms are different, and the quality testing methods used are also different. Therefore, comprehensive and targeted quality testing should be achieved. Taking the quality inspection of granules in solid preparations as an example, the testing content and standards are shown in following table.
| Check items | Standard |
| Appearance | The particles are uniform, without clumping, and the color is consistent. |
| Particle size | The proportion of the total number of test samples that cannot pass through sieve 1 and can pass through sieve 5 is less than 15%. |
| Soluble standard | Soluble particles: 10g of the test sample is dissolved in 200mL of hot water after stirring for 5 minutes;
Effervescent granules: 6 bags are placed in beakers, and 200mL is stirred in water at a temperature of 15~15 ℃ Completely disperse or dissolve in water within 5 minutes. |
| Weight difference | Select 10 bags (bottles) of the test sample, remove the packaging, accurately weigh the actual weight of the contents, and obtain the average filling amount.
It must comply with national regulations on the difference in loading capacity, such as indicating a loading capacity of 1.0g or less, and the difference limit must be within ± 10%. |
Optimize moderate packaging
Optimize Moderate Packaging - Sourced: Complete Packaging Solutions
To achieve sustainable development in the pharmaceutical industry, it is necessary to control the problem of resource waste in solid dosage form production and package appropriately. For example, aluminum-plastic materials are common solid pharmaceutical packaging materials.
When selecting packaging materials in the future, the degree of environmental pollution caused by the materials can be taken into account, and biodegradable and easily treatable materials can be selected to reduce the harm of drug packaging materials to the environment while ensuring that the quality of drugs is not affected.
8.How to Optimize Pharmaceutical Process Technology of Solid Dosage Form?
Taking the common powder in solid dosage form as an example for design, powder dosage form can be obtained by thoroughly mixing powdered drug raw materials and excipients after grinding.
Optimization Of Overall Process For Solid Dosage Form
The advantages of powder are fast effectiveness, fast dispersion speed, and suitability for infants and young children. The overall pharmaceutical process optimization measures for powders require full process quality supervision within the original process, that is, quality control and testing work should be carried out throughout the entire process of drug raw material selection, crushing, mixing, etc.
In addition to ensuring quality supervision and control throughout the entire solid pharmaceutical process, it is also necessary to optimize the technology in the processing stage. For example:
1)Crushing
Various crushing methods such as compression force, bending force, shear force, and impact force are comprehensively applied, and dry, wet, and low-temperature crushing methods can also be selected based on external environmental conditions;
2)Sieving
Strictly following the specifications for drug granules formulated, and use methods such as weaving sieve and punch eye sieve to screen the drug into fine powder and coarse powder as specified in the standards;
3)Mixing
In this stage, some auxiliary materials and additives need to be added, such as flavoring agents, coloring agents, etc. Common mixing methods include diffusion, convection, and shear. Adhesion, fluidity, morphology, and density are all factors that affect the mixing effect. To improve the uniformity of mixing, an optimized grouping and proportioning method can be used;
4)Packaging
Sampling testing methods are often used, and regulations stipulate that the number of problems in the sample size should not exceed 0.01%, otherwise it can be considered that there are many problems in the drug processing process.
9.What Are the the Disposal Method of Solid Dosage Form Waste?
Solid dosage forms inevitably generate waste during the production process, and advanced solid waste treatment technologies should be comprehensively applied in the treatment of solid dosage form waste.
Comprehensive processing technology
Amino acid fermentation residue can be used as organic fertilizer raw materials and feed additives; Vitamin fermentation residue can also be used as feed additive raw materials after efficient dehydration and drying; The drug residue generated in the production of extracted drugs can act as a solvent for water and be used as a raw material for organic fertilizers.
Fermentation residue treatment method
Fermentation Residue Treatment Method - Sourced: AZoLifeSciences
Strictly in accordance with national regulations, culture medium waste generated during the production of hypoglycemic drugs, biotechnology synthesis of amino acids, and statin lipid-lowering drugs must be included in hazardous waste disposal.
Other types of bacterial residues can be used as raw materials for single-cell protein production. Encourage pharmaceutical companies to actively carry out innovative research on comprehensive utilization and harmless treatment of antibiotic residues, such as researching technologies for extinguishing fires and drying residues.
Sludge treatment method
Sludge Treatment Method - Sourced: Healthcare Radius
In dealing with the problem of residual sludge after wastewater treatment in solid dosage form production, sludge dewatering techniques such as concentration, vacuum dewatering, and drying can be used. Concentration combined with general pressure filtration technology and drying technology can be used to reduce the initial moisture content of over 99% to around 97%. After pressure filtration and drying, the moisture content can be directly reduced to around 20%.
Conclusion
Solid dosage forms are a common form of medication due to their good stability, low production and manufacturing costs, and convenient packaging, transportation, and use. After reading this article, you must have learned about the process technology of four common solid dosage forms, as well as common problems and optimization measures. If you have any further questions, please feel free to contact AIPAK at any time.
Don't forget to share this post!
Bin Mixer Related Posts
Bin Mixer Related Products
Bin Mixer Related Videos
CONTACT US
Tell us your raw material and project budget to get quotations within 24 hours.
WhatsApp Us: +86 181 6426 8586
Want the best price & newest pharmaceutical machinery buying guide,tips and trends sent straightly to your box?Sign up for AIPAK’s monthly newsletter,we’re free for your consultation and Offer you the most suitable solutions!
The Buyer's Guide
- Capsule Filling Buyer's Guide
- Blister Packaging Buyer's Guide
- Tablet Counting Buyer's Guide
- Tube Filling Buyer's Guide
- Cartoning Buyer's Guide
- Gummy Making Buyer's Guide
- CO2 Extraction Buyer's Guide
- Empty Capsules Buyer's Guide
- Suppository Filling Buyer's Guide
- Tablet Coating Buyer's Guide
- Tablet Press Buyer's Guide
- Softgel Encapsulation Buyer's Guide
Most Popular
- 7 Importance Of Pharmaceutical Packaging In Different Applications You Must Know
- 6 Advantages You Must Know About Tablet Counting Machine
- 8 Advantages of Blister Packaging You Must Know
- 6 Critical Applications of Automatic Capsule Filling Machine
- 6 Stations You must Know to Improve the Filling Quality of Automatic Capsule Filling Machine