Semi-solid Pharmaceutical Manufacturing & Handling: The Complete FAQ Guide In 2025
Do you need guidance and professional knowledge for the production of semi-solid dosage forms? Are you looking for a professional and experienced semi-solid dosage form manufacturer and partner? Are you confused about the material formulation development and testing of semi-solid dosage forms?
Even if you have not found a partner, through this post, you may have a good understanding of the production process and related expertise of semi-solid dosage forms. This complete FAQ guide covers all stages and process information of all semi-solid production and manufacturing. Check it out now!
1.What Is Semi-solid Dosage Form?
What Is Semi-solid Dosage Form-sourced: shopmaryann
The semi-solid dosage form is a drug or product that is between solid and liquid. It is mainly used for smearing and external application, and can be applied to a wide range of areas, including the skin, nasal cavity, cornea, rectum, vagina, mouth, ear or urethral membrane. Because it needs to be applied directly to the skin, the semi-solid dosage form has a smooth texture and no gritty feeling, and does not cause any irritation to the skin.
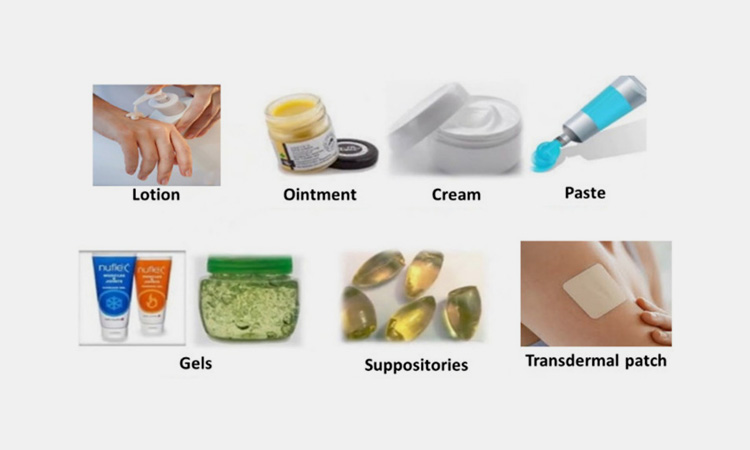
2.What Are The Types Of Semi-solid Pharmaceutical Manufacturing & Handling Products?
The semi-solid dosage form products are very diverse. They mainly include:
Ointments
Ointments-sourced: hapdco
Ointments are thicker than creams and lotions. They are usually packaged in soft tubes for easy access. The production process of ointments requires the addition of more oily bases. This will provide more sealing effects to the parts that need to be applied.
Creams and lotions
Creams and lotions-sourced: healthline
Creams and lotions are slightly thinner than ointments. Generally, their water content is higher than their oil content. This makes their texture softer and easier to spread. Therefore, creams and lotions are often packaged in bottles or cans.
Pastes
Pastes-sourced: drbicuspid
Pastes have a higher viscosity. They are mainly a mixture of powder and ointments. They are very common in the food, chemical and pharmaceutical industries. Pastes have very low water content and no fluidity. They are often packaged in cans and bags that are easy to access.
Gels
Gels-sourced: biopac
Gels are a water-soluble colloidal suspension semisolid solution, and often exist in a transparent semi-liquid form. Its soft and slippery consistency allows this semi-solid to be easily spread on your skin. During the manufacturing process, more water is added.
Jellies
Jellies-sourced: xylocaine
Jellies have the same consistency as gels. They are mainly composed of water and active ingredients, and a little bit of gelling agent. They are widely used in the food industry.
Poultices
Poultices-sourced: argileduvelay
Poultices are pastes for external use only. In the pharmaceutical industry, they are often in the form of ointments.
Suppositories
Suppositories-sourced: anusol
Suppositories are semi-solid preparations designed to be inserted into the vagina or rectum. They can be dissolved or melted at body temperature, and then release the active ingredients until they are absorbed by the skin in the rectum or vagina.
3.Why Is Semi-solid Pharmaceutical Manufacturing & Handling Important?
Semi-solid pharmaceutical manufacturing and handling can provide you with good products and effects.
Versatility
Versatility-sourced: argileduvelay
Semi-solid pharmaceutical manufacturing and handling can formulate various active substances and APIs with targeted effects into semi-solid forms to open up new treatment methods and effects for you, adapting to different patients and different needs.
Targeted drug delivery
Targeted drug delivery-sourced: rodpub
Semi-solid processing and manufacturing can achieve the effect of precise targeted drug delivery, and can handle some situations that conventional drugs or methods cannot handle well.
Provide professional semi-solid product solutions
Provide professional semi-solid product solutions-sourced: contractpharma
Semi-solid products are used in a wide range of industries and groups, and different groups have different functional requirements for products. The good semi-solid processing and manufacturing can provide you with more professional semi-solid production solutions.
4.What Are The Main Equipment Applied For Semi-solid Pharmaceutical Manufacturing & Handling?
Able to manufacture and handle the semi-solid materials, you need to make good use of the machines and equipment below:
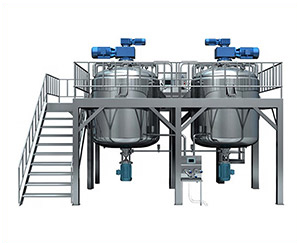
Vacuum emulsifying mixer
AIPAK vacuum emulsifying mixer
Vacuum emulsifying mixer is a kind of equipment specially used for mixing, dispersing, homogenizing, emulsifying liquids, semi-liquids, oily substances and semi-solids. It has a vacuum system, a heating system, and a cooling system, and is suitable for almost all semi-solid materials, including ointments, creams, lotions, gels, pastes, and more.
Working principle of vacuum emulsifying mixer:
Working principle of AIPAK vacuum emulsifying mixer
- Manual feeding, add the materials to be processed and mixed into the water pot and the oil pot;
- The materials will be heated, stirred and evenly mixed in the water pot and the oil pot;
- The vacuum pump will suck the evenly stirred mixture into the emulsifying pot;
- The scraper in the emulsifying pot will continuously stir the mixture, and the electric heating tube will continuously heat it, so that the material is continuously sheared and impacted;
- The material is quickly broken and cut during the shearing process;
- The bubbles generated in the vacuum pot are also taken away in time;
- All materials are micronized, emulsified, mixed, homogenized, dispersed, etc. in a short time.
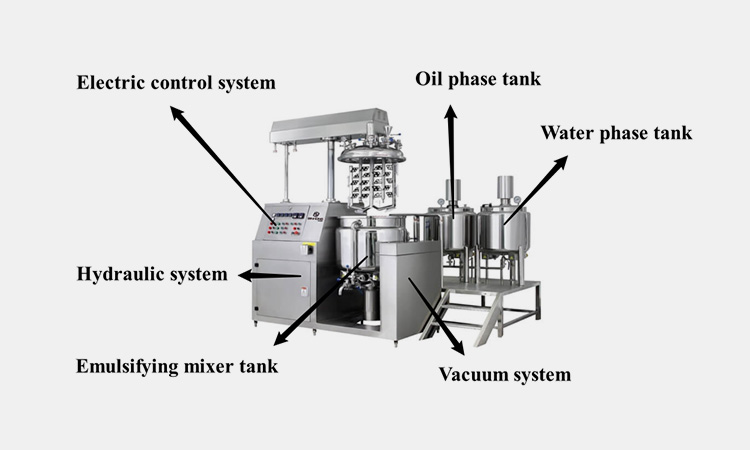
Main parts of vacuum emulsifying mixer:
Main parts of vacuum emulsifying mixer-sourced: ginhong
Emulsifying mixer tank
Emulsifying mixer tank generally adopts a three-layer structure, a cutting vortex emulsification structure, a low-speed frame scraping structure, and a low-speed reverse stirring structure.
Oil phase tank
Oil phase tank is mainly used to process oil mixture.
Water phase tank
Water phase tank is mainly used to store and process the water needed in the mixture.
Vacuum system
Most vacuum emulsifying mixers use water ring vacuum pumps. It can compress and extract the air in the tank to prevent bubbles from being generated during the emulsification process.
Hydraulic system
Hydraulic system is generally composed of oil cylinder, solenoid valve, overflow valve, oil tank, pressure maintaining valve, etc.
Electric control system
Vacuum emulsifying mixer uses a high-end electrical control system.
Tube filling machine
AIPAK tube filling machine
Tube filling machine is a kind of equipment specially used to pack various ointments, creams, lotions, gels, pastes into tubes. In addition to solid materials, it is suitable for filling and sealing of almost all materials.
Working principle of tube filling machine:
Working principle of AIPAK tube filling machine
- Manual tube feeding. You need to put your soft tubes into the tube hopper in advance to facilitate the subsequent sorting and filling;
- Automatic tube sorting. When your soft tubes are fed in, the tube rack and the rotating table will rotate together to sort the tubes, and then proceed to the following steps;
- Automatic tube feeding. The sorted tubes will be sent to the filling station;
- Tube filling. When the soft tubes arrive at the automatic filling station, the empty tubes will automatically fall into the tube rack. The material will be directly filled into the empty tube from the filling nozzle;
- Tube sealing. The filled soft tubes will be marked and positioned, and then heat-sealed;
- The sealed asymmetrical or uneven ports will be trimmed again;
- Coding and batch printing. The corrected finished product will be coded and printed;
- The tubes without any problems will be ejected for further packaging, etc.;
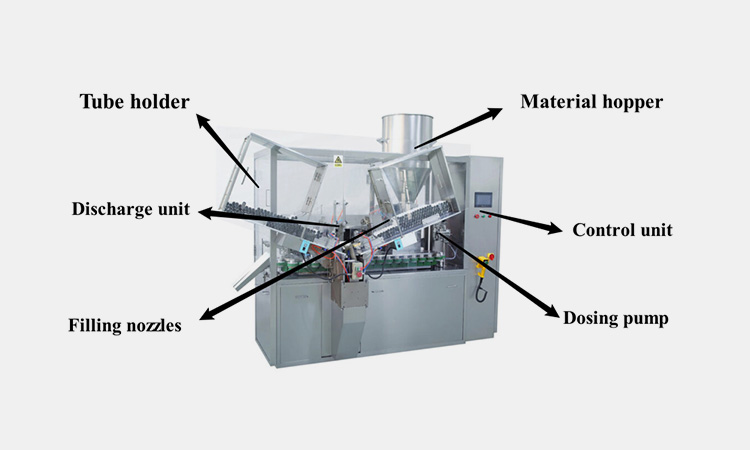
Main parts of tube filling machine:
Main parts of AIPAK tube filling machine
Material hopper
The material hopper is used to store the material to be filled.
Filling nozzles
The filling nozzles are used to adjust the filling flow of the material to avoid leakage and waste.
Dosing pump
The dosing pump can accurately fill different doses of material into the appropriate tubes by pneumatic means.
Discharge unit
It is responsible for controlling the filling nozzles to evenly distribute the material into the packaging hose. It can correctly identify the correct position and related capacity of the tube.
Tube holder
It is used to hold the empty tubes that need to be filled. It can adjust the space according to your needs to increase the number of empty tubes.
Control unit
The control unit is used to set parameters and adjust the speed of the equipment.
Suppository filling machine
AIPAK suppository filling machine
Suppository filling machine is specially used for making rectal and vaginal suppositories. Such suppositories are usually oval, bullet, torpedo, duckbill, etc., and will be dissolved and absorbed when heated in the vagina. This equipment can meet your production needs of various suppository shapes. It is equipped with heat preservation and heating function, which can ensure the fluidity and activity of the liquid during production.
Working principle of suppository filling machine:
Working principle of AIPAK suppository filling machine
- Film unwinding. You can choose PVC, PE or PVC+PE type film materials as the outer packaging material. When the machine starts, the film will start unwinding. The film will be transferred to the molding part.
- Molding and formation. The film will enter the molding module through the guide wheel. According to the mold you choose, your film will be preheated, heated, molded and blown.
- Material filling. The processed material will be sent to the filling station. It can achieve heating and filling, keep the material at the right temperature all the time, and facilitate filling accuracy.
- Suppositories cooling. The filled suppositories will be sent to the cooling tube and the temperature will be controlled between 8-16 degrees Celsius to wait for the suppositories to cool and solidify.
- Suppositories sealing. The cooled and solidified suppositories will be sent to the tail seal mold. The pneumatic sealer will seal the tail of the suppository.
- Printing and coding. The packaged suppositories will be sent to the coding and printing stage.
- The printed costs will be sent to the cutting section. The cutter will cut the serial suppositories separately to facilitate the subsequent packaging.
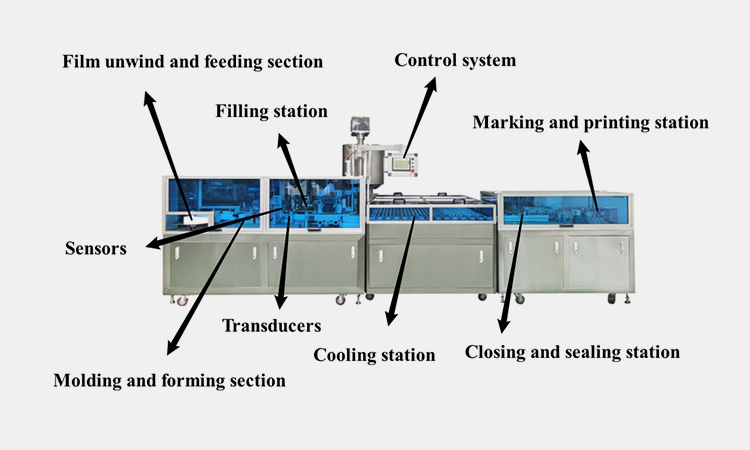
Main parts of suppository filling machine:
Main parts of AIPAK suppository filling machine
Film unwind and feeding section
The film unwind and feeding section is mainly responsible for the unfolding, delivery and reception of the film, which is convenient for the molding of the subsequent material packaging shell.
Molding and forming section
The film enters the molding and forming section part along the guide rail and will undergo heating, forming, blow molding and other steps to facilitate the filling of the subsequent materials.
Filling station
Filling station is equipped with material barrel, agitator and circulation pump. It can transfer the processed materials to filling hopper through pressure pump and then distribute them into the blown suppository shell.
Cooling station
Cooling station is mainly composed of cooler, cooling fan and cooling tunnel. It can help suppository to cool down and solidify quickly in a short time without affecting the active ingredients in the preparation.
Closing and sealing station
The tail of the cooled and solidified suppository will be pneumatically sealed.
Marking and printing station
According to your needs, you can print the batch number, production date and manufacturer in the appropriate position.
Control system
Control system adopts PLC system and HMI system. It can help read various information you set and then perform trial identification to control the machine according to your requirements.
Sensors and transducers
Suppository filling machines generally use ultrasonic, temperature, piezoelectric and pressure sensors.
5.What Are The Development Process Of Semi-solid Pharmaceutical Manufacturing & Handling?
If you want to have a good semi-solid pharmaceutical manufacturing and handling process, you can follow the steps below:
Formula design
Formula design-sourced: vicihealthsciences
According to your own needs and the symptoms you want to solve, while considering the physical and chemical properties of the product and complying with relevant laws and regulations, you can develop and design a product with good stability and strong functionality.
Equipment preparation
Equipment preparation-sourced: contractpharma
In the early stage, you may need equipment for liquid mixing, solid mixing, semi-solid mixing and dispersion, solid grinding equipment, emulsification equipment, homogenizer, ultrasonic equipment, etc. in the middle stage, and filling equipment, packaging equipment and printing equipment in the later stage.
Filling and packaging operation
Filling and packaging operation-sourced: nutraceuticalsworld
After the raw materials are processed by the filling and packaging machines under a strictly evaluated and controlled environment, you can start preparing for the filling and packaging steps. Try to control the consistency, uniformity and stability of the product.
Product testing
Product testing-sourced: andreasastier
In order to check whether the finished product meets the standards and whether it can be put on the market later, you need to conduct product testing. You can collect several batches of samples separately and conduct random inspections later.
Batch sampling
In different batches, you can select different quantities of samples to test the stability, uniformity, efficacy, composition, etc. of the samples, and keep records for later analysis and summary.
6.What Are The Key Factor For Semi-solid Pharmaceutical Manufacturing & Handling?
The most critical factors in the production of semi-solids are:
Temperature
When dealing with semi-solid materials, temperature is a factor that needs to be paid attention to at all times. Especially when dealing with semi-solid mixtures of oils and water, you need to control the temperature well in order to optimize its uniformity, particle distribution and physical properties of spreadability.
| Items | Temperature requirements |
| Cream
|
When dealing with cream materials, the temperature needs to be controlled at around 70 degrees Celsius; |
| Gel | To improve the stability of gel, you need to control the temperature at around 4 to 10 degrees Celsius; |
| Ointment | To improve the stability of ointment, you can increase the temperature to 40 degrees Celsius, or between 30 degrees Celsius and 80 degrees Celsius; |
Equipment
When producing semi-solid products, it is also important to choose suitable and excellent equipment. These include agitators, emulsifiers, jackets, storage tanks, mixers, grinders, etc., all of which are indispensable.
Testing
Testing is also one of the most important links in the entire production process. In fact, you can conduct tests around the physical, microbiological and chemical aspects of the product. Determine the product's color change, pH value, crystallization, and appearance, viscosity or spreadability requirements.
Packaging and storage
cphi-online-sourced: cphi-online
Packaging and storage can determine the final effect of the product. You need to pay special attention to whether different products are packaged and stored in the correct or appropriate way.
7.What Are The Critical Process Parameters During Semi-solid Pharmaceutical Manufacturing & Handling?
During the production process, special attention should be paid to the heating and cooling of materials, mixing methods and speeds, pressure differences in the tank, and the use of tools.
Heating and cooling
Too high a temperature will cause the active substances in the product to lose their activity, increase the concentration of the product, and affect the stability of the product. Too low a temperature will cause the ingredients to precipitate and solidify at any time, affecting the quality of the product.
Mixing method and speed
Mixing method and speed-sourced: fatdaddios
Incorrect mixing methods and speeds will also affect the quality of the product. In order to improve the homogenization and dispersion of the product, control the viscosity of the product, and master the shear degree and shear amount for different products.
Pressure difference in the tank
After determining the shear amount and shear force, you need to adjust the pressure difference in the tank. Control the measuring device and display of the vacuum pressure gauge, etc., and make adjustments in time.
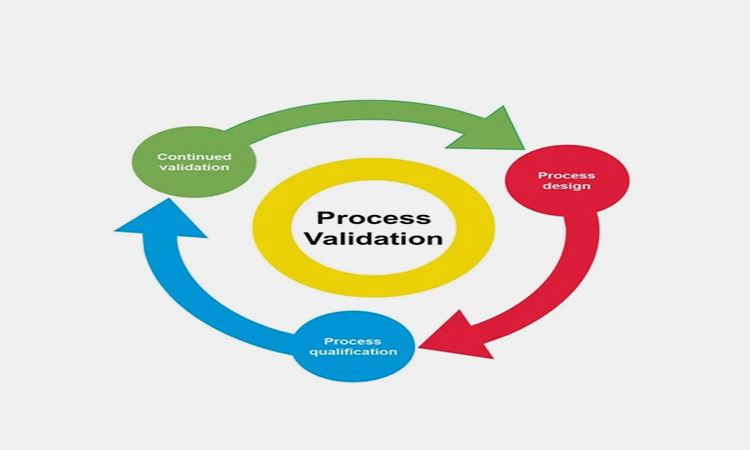
8.What Are The Semi-solid Pharmaceutical Manufacturing & Handling Process That Should Be Validated?
The semi-solid pharmaceutical manufacturing and handling process that shall be validated is:
Cleaning
Cleaning-sourced: Aipuweier
This includes environmental cleaning, equipment cleaning, tool cleaning and material cleaning, all of which are indispensable to ensure that the materials are not contaminated during the handling process.
Disinfection
The cleaned equipment, tools and the overall environment are disinfected to ensure that the materials are not contaminated from the outside.
Fumigation
The disinfectant in the equipment is removed by fumigation to further improve the sterility of the entire process.
Sterilization
After handling the materials, the hoses, bottles or cans to be filled are sterilized to prevent the products from being contaminated by the internal environment after filling, which affects the overall quality of the products.
Aseptic filling
Aseptic filling-sourced: apacks
Through the aseptic filling process, the products are ensured to be filled into different packaging shells intact, which is convenient for the use and treatment of patients later.
Sealing and storage
Using different packaging methods, the products are packaged by different sealing methods, and then stored at low temperature or room temperature in the dark as required.
9.What Are The Protocols That Semi-solid Pharmaceutical Manufacturing & Handling Process Needs?
The protocols that you may need to follow about the semi-solid pharmaceutical manufacturing and handling process are:
Protocols That Semi-solid Pharmaceutical Manufacturing & Handling Process Needs-sourced: jsapharmaguideline
- The purpose and premise of your production of semi-solid products;
- Introduction to the entire process of semi-solid pharmaceutical manufacturing and handling, including the equipment used, flow charts, key steps, details that need attention, etc.;
- Approval reports, relevant qualifications for installation, production and operation, etc.;
- Your series of semi-solids processing related methods, procedures, steps, standards, test processes, test data, result summaries, etc.;
- Product batch sampling and data inspection and testing;
- Related verification and certification reports.
10.What Are The Challenges And Innovations For Semi-solid Pharmaceutical Manufacturing & Handling?
Although the current semi-solid pharmaceutical manufacturing and handling technology is relatively mature, it still faces many challenges. These include:
Development of other semi-solid dosage form types
Development of other semi-solid dosage form types-sourced: renejix
The development of other types of semi-solid formulations involves API separation of various excipients, extraction of high-tech biological ingredients, design and development of new formulations, and development and design of various sensitive formulations.
Transdermal semi-solids development
Skin is the body's natural barrier. To deliver API ingredients in transdermal preparations into the blood, better solutions and designs are necessary.
Reduce side effects
Reduce side effects-sourced: is
Products with significant effects and high functionality inevitably produce varying side effects. Balancing the advantages and disadvantages of good effects and side effects is also one of the challenges.
11.What Are The Future Trends For Semi-solid Pharmaceutical Manufacturing & Handling?
In the future, semi-solids pharmaceutical manufacturing and handling technologies will also change. They are:
Smart formula
Smart formula-sourced: optik-teske
According to the needs of different patients or users, smart formulas can adjust viscosity, active ingredients, etc. at any time, and can release different active substances according to different needs to adapt to a wider range of needs.
Eco-packaging
Eco-packaging-sourced: thepackagingcompany
The future world must be developing towards a more environmentally friendly packaging method. With the development of society, manufacturers will adopt more environmentally friendly packaging materials to adapt to the development of the environment.
Customized service
In the future, there will be more customized formulas and product production services. It can formulate slightly different products according to the needs of different users to adapt to more personalized requirements.
Conclusion:
Through the study and understanding of the complete FAQ guide of semi-solid pharmaceutical manufacturing and handling, to prepare successful semi-solid products, you need to pay attention to different details and requirements. If you have more research on semi-solid manufacturing and processing, you are welcome to consult AIPAK now!
Don't forget to share this post!
Vacuum Emulsifying Mixer Related Posts
Vacuum Emulsifying Mixer Related Products
Vacuum Emulsifying Mixer Related Videos
CONTACT US
Tell us your raw material and project budget to get quotations within 24 hours.
WhatsApp Us: +86 189 7157 0951
Want the best price & newest pharmaceutical machinery buying guide,tips and trends sent straightly to your box?Sign up for AIPAK’s monthly newsletter,we’re free for your consultation and Offer you the most suitable solutions!
The Buyer's Guide
- Capsule Filling Buyer's Guide
- Blister Packaging Buyer's Guide
- Tablet Counting Buyer's Guide
- Tube Filling Buyer's Guide
- Cartoning Buyer's Guide
- Gummy Making Buyer's Guide
- CO2 Extraction Buyer's Guide
- Empty Capsules Buyer's Guide
- Suppository Filling Buyer's Guide
- Tablet Coating Buyer's Guide
- Tablet Press Buyer's Guide
- Softgel Encapsulation Buyer's Guide
Most Popular
- 7 Importance Of Pharmaceutical Packaging In Different Applications You Must Know
- 6 Advantages You Must Know About Tablet Counting Machine
- 8 Advantages of Blister Packaging You Must Know
- 6 Critical Applications of Automatic Capsule Filling Machine
- 6 Stations You must Know to Improve the Filling Quality of Automatic Capsule Filling Machine