What Is The Humidity Requirements For Pharmaceutical Industry
Whenever or whatever you want to produce in pharmaceutical industry, there are humidity requirements for environmental conditions for production. During the drug production process, humidity that is too low or too high can have an adverse effect on the pharmaceutical products.

Humidity Requirements For Pharmaceutical Industry-sourced: pulse
However, what is the humidity requirements for pharmaceutical industry in details? And what are their effects? Why does the humidity in the medicine storage room need to be maintained at the optimal environmental level? How to control the humidity of the environment? Let's learn about it together!
1.What Is The Humidity Requirements For Pharmaceutical Industry?
As the technology of the pharmaceutical industry continues to develop, the production environment conditions are more and more strict, especially the requirements and control of humidity. Humidity is the amount of water vapor present in the air. The temperature and pressure can affect the change of humidity.
Humidity Requirements For Pharmaceutical Manufacturing
Humidity Requirements For Pharmaceutical Manufacturing-sourced: translimite
Powders, tablets & capsules quality and shelf life may be severely reduced when they get in contact with airborne water vapor. Their humidity range requires usually around ±2%RH and ±1ºC. Poor humidity environment control will directly affect the pharmaceutical products production line.
Humidity Requirements For Labors In Pharmaceutical Production
Humidity Requirements For Labors In Pharmaceutical Production-sourced: translimite
The humidity for labors in pharmaceutical production workshops is to provide comfortable controlled conditions for employees with good efficiency. The usual temperature range is around 18ºC to 23ºC, and the humidity range is 40% to 60% relative humidity (RH).
Humidity Requirements For Clean-rooms
Humidity Requirements For Clean-rooms-sourced: techconversions
The clean-room is used for controlling environmental variables and provides a high-quality environment for the research and development and manufacturing of your pharmaceutical products. The clean-room can prevent contaminants such as pathogens, dust, viruses and bacteria.
Humidity Requirements For Laboratories
Humidity Requirements For Laboratories-sourced: freshliance
The precise humidity requirements for laboratories is vital for production yields and minimise waste control. The different humidity controls will affect the fast drying of tablet coatings and printing problems.
2.Why You Need To Follow Humidity Requirements For Pharmaceutical Industry?
Most pharmaceutical products contain active pharmaceutical ingredients (API), filling powders, capsules, tablets, etc., which are hygroscopic. To follow the humidity requirements is important.
Ensure Pharmaceutical Products Quality
Ensure Pharmaceutical Products Quality-sourced: tipt
More or less moisture may destroy or degrade the active pharmaceutical ingredients in the medicines. By controlling the humidity in the pharmaceutical process, the migration of moisture may be prevented, thereby ensuring the effectiveness of the pharmaceutical products.
Maintain Pharmaceutical Products Shape
Maintain Pharmaceutical Products Shape-sourced: vintura
When filling and packaging pharmaceutical powders, if the humidity is too high, lumps will form and the powder will stick to the conveyor belt. It will cause the product to have different shapes, thus affecting the quality of pharmaceutical products.
Improve Process Efficiency and Output
Improve Process Efficiency and Output-sourced: qualityze
Maintaining the right humidity level can ensure consistent pharmaceutical material flow, granulation, drying and packaging, thereby it can reduce the batch failures, increasing output, reducing costs and improving your productivity.
Keep the Integrity of the Packaging
Keep the Integrity of the Packaging-sourced: news-medical
The storage and logistics of medicines also require humidity control to ensure the integrity of chemical and pharmaceutical products and keep your pharmaceutical products packaging and labels intact.
Assure Ideal Storage Humidity
Assure Ideal Storage Humidity-sourced: sannova
Excessive humidity can cause mold growth. To prevent the drug from deteriorating during storage, it is important to maintain the ideal storage humidity for the drug. The temperature range is 15° to 25° C and the relative humidity should be less than 60% (RH).
Inhibit Microbial Growth
Inhibit Microbial Growth-sourced: solutionpharmacy
Humidity affects the growth of microorganisms. Molds and mildew thrive more easily in higher humidity. Humidity also affects the ability of the environment to support the growth of any viable contaminants.
Bring Pharmaceutical Worker Comfort
Bring Pharmaceutical Worker Comfort-sourced: processingmagazine
It is also very important to assure the operator a comfortable environment during the pharmaceutical manufacturing process. By creating comfortable humidity and controlled conditions, the speed and efficiency of product production can be maintained.
Good Manufacturing Practices
Good Manufacturing Practices-sourced: raiselabequip
In the pharmaceutical manufacturing process, it is essential to control humidity in all aspects of production, storage, testing, mixing and packaging. The equipment, personnel, processes and environment in the pharmaceutical process must be maintained within the specified humidity range.
3.What Are The Factors You Need To Consider Of Humidity Requirements For Pharmaceutical Industry?
What are the specific factors that would influence humidity requirements and control? They are:
Operator Comfort
Operator Comfort-sourced: sensoscientific
When considering about the operator comfort in the pharmaceutical industry, they including your metabolic rate, amount of clothing, temperature, skin condition (humidity), personal preference, etc. that all need to be taken into account.
Environmental Control
Environmental Control-sourced: kirinoikeuchi
If the humidity of the environment is not well controlled, it will directly affect the pharmaceutical production line. If the pharmaceutical humidity is lower than 45%RH, it will cause static charge accumulation, which will eventually cause the product to become dry and of poor quality.
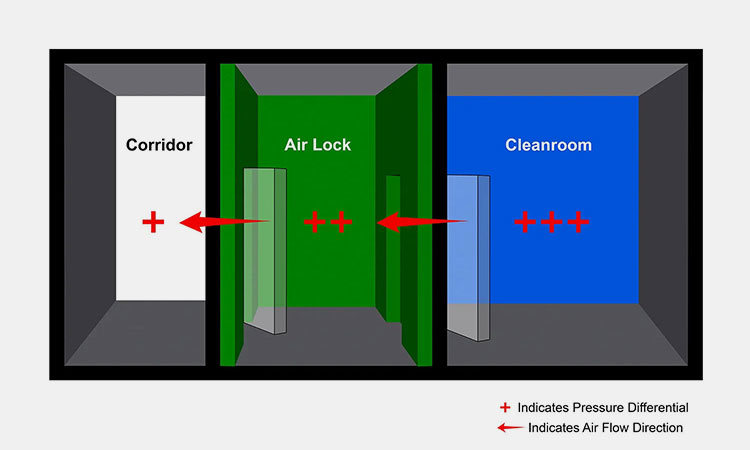
Clean-room Control
Cleanroom Control-sourced: cleanroom-industries
Clean-room is the best means of environmental control. It not only provides a good operating environment for operators, but also provides a good sterile environment for pharmaceutical production. When temperature and humidity are controlled, pharmaceutical production can ensure quality.
Microbial Growth
Humidity and temperature are a consideration for microbial growth as it affects microbial growth and spore germination. When humidity is not properly controlled, bacterial reproduction rates roughly double.
Water Activity
Water activity is the ratio of the vapor pressure in the product to the vapor pressure of pure H2O at the same temperature. Changes in water activity can also accommodate changes in humidity.
4.What Are The Humidity Range Recommendations In Pharmaceutical Industry?
Humidity Range Recommendations-sourced: humiscope
If you are a company specializing in pharmaceutical manufacturing, processing and storage, maintaining strict hygiene standards and humidity control is key to ensuring high-quality products. So, the humidity range recommendations in pharmaceutical industry are:
Table 3 Optimal Humidity Levels for Pharmaceutical Manufacturing
| No. | Stages | Humidity Range | Details |
| 1 | Formulation stage; | l Humidity range is 30-40%RH; | l During the formulation process, too high humidity can cause certain drug substances to agglomerate, clump or chemically degrade; |
| 2 | Granulation stage; | l Humidity range is between 40-50%RH; | l During the granulation stage, too high or too low humidity can cause static charge, affecting powder flow and uniformity;
l Ultimately leading to poor product quality; |
| 3 | Packaging stage; | l The optimal packaging humidity range is usually around 40-50%RH; | l High humidity levels can lead to degradation, loss of efficacy and shortened shelf life;
l Low humidity levels can cause packaging materials to become brittle and crack; |
| 4 | Storage stage; | l The ideal storage humidity range is usually 30-60%RH between; | l Humidity control is essential to ensure the integrity, stability and high quality of drugs;
l High humidity can affect the risk of drug microbial growth and affect the effectiveness of preservatives; l Low humidity can cause water loss, which can affect changes in physical properties; |
Table 4: Humidity Range Recommendations For Pharmaceutical Facility
| No. | Projects | Humidity Range |
| 1 | Colloids; | 30 to 50% RH |
| 2 | Cough drops; | 40 +/-3%RH |
| 3 | Ampoule manufacturing; | 35 to 50%RH |
| 4 | Serums and animal rooms; | 50+/-1%RH |
| 5 | Plant utilities room | 80 to 90% RH |
| 6 | Clean utilities | 60 to 65%RH |
| 7 | Raw materials warehouse | 20 to 80%RH |
| 8 | Finished goods(refrigerated) | 20 to 80%RH |
| 9 | Dry Storage | 30 to 40%RH |
Regarding humidity control, the US and the EU have different detailed standards:
Table 5: US Regulation about Humidity Control
| No. | Items | Details |
| 1 | Operating range; | l Separate areas or other control systems should be used to control humidity levels; |
| 2 | Humidity control equipment; | l Equipment should be provided to adequately control air pressure, microorganisms, dust, humidity and temperature; |
| 3 | Storage procedures; | l Humidity control conditions and procedures for drug storage should be followed; |
| 4 | Appropriate temperature; | l Drugs should be stored under appropriate temperature, humidity and light conditions to avoid affecting the identity, strength, quality and purity of the drug; |
Table 6: EU Regulation about Humidity Control
| No. | Items | Details |
| 1 | Lighting, temperature, humidity and ventilation; | l You need to control the lighting, temperature, humidity and ventilation conditions to maintain the accurate humidity environment of the drug or equipment during manufacturing and storage; |
| 2 | Storage conditions; | l When designing or renovating the storage area, you need to ensure good storage conditions; |
| 3 | Clean area; | l When staff need to wear sterile clothing, the humidity and temperature environment must maintain a comfortable environment; |
5.What Are The Adverse Effects Of Humidity In Pharmaceutical Industry?
There may be adverse effects of humidity in pharmaceutical industry, and they are
Adverse Effects Of Humidity Level On Tablet Stability
Adverse Effects Of Humidity Level-sourced: madgetech
Humidity level has a great impact on the stability of tablets. Therefore, during the packaging process, manufacturers generally use blister, capsule shell or bottle packaging to prevent moisture. If the humidity level cannot be controlled, the water content of tablets increases, resulting in drug exfoliation.
Table 1: Adverse Effects Of Humidity Level On Tablet Stability
| No. | Adverse Effects | Details | Reason |
| 1 | Hardness decreases; | l At 75% relative humidity, the hardness of tablets decreases; | l Under high humidity, tablets absorb moisture, resulting in a decrease in tablet hardness; |
| 2 | Stability decreases; | l At 25 degrees, the hardness of tablets decreases by 5-10%; | l When the humidity is too high, tablets absorb moisture, which will affect physical stability and make the tablets ineffective; |
| 3 | Effect on particle size; | l At 45 degrees, the hardness of tablets decreases by 10-39%; | l Tablets stored under high humidity conditions will have higher viscosity; |
| 4 | Effect on shapes; | l At about 60% relative humidity, the stability of tablets decreases; | l Tablets stored under high humidity conditions have more cracks; |
In addition to affecting drugs or tablets, humidity control is very important in the pharmaceutical industry to maintain the safety and effectiveness of stored drugs. They are:
Table 2: Adverse Effects Of Humidity Level On Pharmaceutical Manufacturing
| No. | Adverse Effects | Details |
| 1 | Shortened shelf life; | l Some drugs with high active ingredients will deteriorate faster under high humidity conditions, thus shortening the shelf life; |
| 2 | Product degradation; | l Drugs containing high protein will cause degradation, instability and even poisoning under high humidity conditions; |
| 3 | Microbial growth; | l When the relative humidity reaches 60% or higher, bacteria, viruses, mold, fungi and mites will grow; |
| 4 | Low product quality; | l Particles in powders and bubbles in injections will seriously affect product quality if affected by humidity; |
| 5 | Equipment damage; | l High humidity environments will damage sensitive and expensive testing equipment; |
6.What Are The Temperature Range Recommendations For Humidity Control In Pharmaceutical Industry?
Temperature Range Recommendations-sourced: aavadinstrument
Humidity control plays a key role in maintaining the efficacy and stability of drugs, especially those that are sensitive to humidity. Accurate humidity control can maintain the chemical stability of drugs. Temperature is a key indicator for maintaining humidity:
Table 7: Temperature Range Recommendations In Pharmaceutical Manufacturing
| Production Process | Temperature | Humidity |
| Weighing & Mixing | 68 to 72°F (20 to 22°C) | 35 to 40% RH |
| Compression | 68°F (20°C) | 25 to 35% RH |
| Pan Coating | 53 to 203°F (12 to 95°C) | 10 to 70% RH |
| Filling and Packing | 68°F (20°C) | 10 to 35% RH |
| Storage | 68 to 77°F (20 to 25°C) | 45% RH |
7.How To Control The Humidity In Pharmaceutical Manufacturing?
Humidity Control In Pharmaceutical Manufacturing-sourced: ispe
There are several ways for you to control humidity:
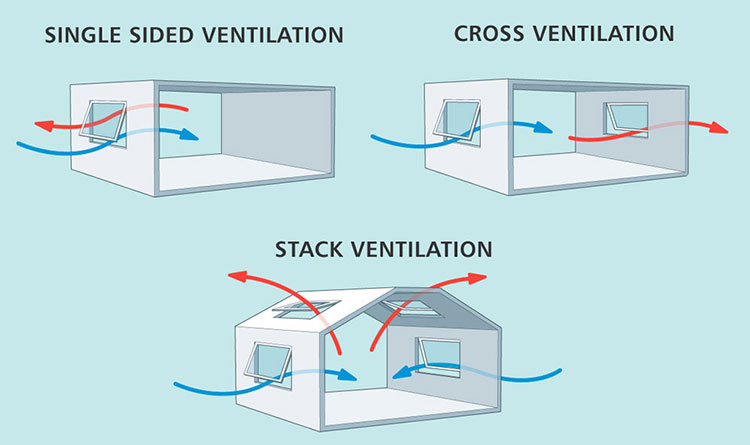
Natural Ventilation
Natural Ventilation-sourced: tealproducts
Using outdoor air for ventilation is an effective way to control humidity in a building. However, ventilation air must have a lower moisture content than the air inside the building to be effective. Also, this method is very affected by the type of pharmaceutical and changing weather and seasonal conditions.
Desiccant Dehumidifiers
Desiccant Dehumidifiers-sourced: odellassoc
Desiccant dehumidifiers reduce relative and absolute humidity and also reduce dew point. They are suitable for working environments between 100°F and -40°F (40°C and -40°C). It is very effective in reducing environmental humidity and maintaining equipment performance and extending equipment life.
Dehumidifiers or Humidifiers
Dehumidifiers or Humidifiers-sourced: level-solutions
You can use a variety of dehumidifiers or humidifiers to control humidity in pharmaceutical manufacturing facilities and environments. Dehumidifiers use refrigeration to remove excess moisture from the air. Humidifiers use steam to increase the humidity in the air.
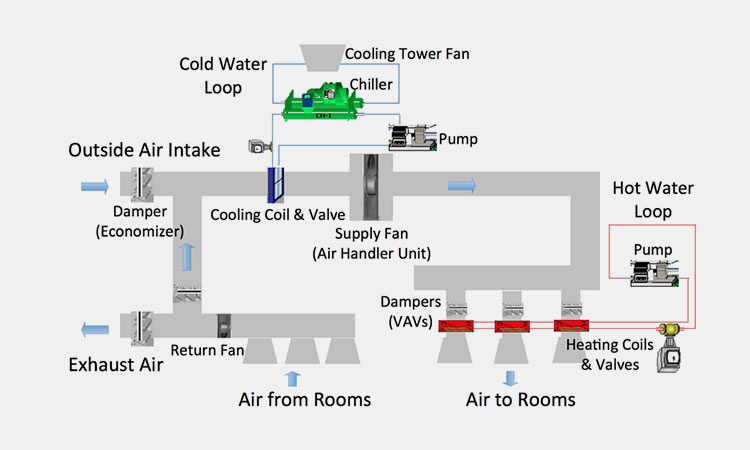
HVAC System
HVAC System-sourced: researchgate
The HVAC system stands for heating, ventilation and air conditioning, and it can provide constant humidity for your entire environment through air conditioners, heat pumps, refrigerants, air handlers, thermostats and temperature agents.
Barrier System
Barrier System-sourced: terrauniversal
The barrier system mainly uses isolator or barrier technology to provide a suitable or constant humidity controlled environment for a specific manufacturing process or equipment.
Humidity Sensor
Humidity Sensor-sourced: setra
The humidity sensor can provide real-time industrial environment humidity levels. It can closely monitor the temperature and humidity conditions in the production environment to provide you with efficient pharmaceutical production levels.
8.What Is The Humidity Monitoring In The Pharmaceutical Industry?
In addition to humidity control techniques, humidity monitoring is also something to consider when storing medications. You need to make sure that the temperature and humidity are regulated correctly.
Humidity Monitoring
Humidity Monitoring-sourced:atlas-scientific
The World Health Organization has set out optimal conditions related to humidity monitoring. For optimal storage of medications, the relative humidity level should be 60% or less. There are a number of practices you can follow to effectively maintain and monitor humidity levels.
Practices For Humidity Monitoring
Practices For Humidity Monitoring-sourced: vaisala
There are three best practices for you to keep the humidity monitoring, including setting up separate sealed areas, preventing moisture migration, removing and adding moisture, etc.
Setting up separate sealed areas
If medications require low humidity storage conditions, you can set up a separate area for medication storage and ensure that they are separated from other areas.
Preventing moisture migration
If you are using an HVAC system, when monitoring humidity, you need to ensure that moisture does not migrate through the HVAC system into other systems.
Removing and adding moisture
When you set the humidity to the appropriate level, if the humidity is still too low or too high, you need to take measures to increase the required humidity level or reduce the excessive humidity level.
Use a humidity detection system
By utilizing a humidity detection system, you can more easily and effectively track humidity conditions, obtain real-time data, and make corresponding adjustments, etc. It can help you automatically record humidity data, maintain humidity system safety, etc.
9.What Are The USP 797 And USP 800 Guidelines Of Humidity Requirements For Pharmaceutical Industry?
USP is a not-for-profit, science-driven organization that formulates the process for healthcare quality standards. The USP 797 and 800 guidelines provide standard guidelines for humidity and temperature control in the pharmaceutical industry.
USP 797
USP 797-sourced: hardydiagnostics
USP 797 covers humidity control for personnel, engineering/facility design, and environment. Its purpose is to provide requirements for each area to ensure safe, sterile drug preparation.
USP 800
USP 800-sourced: hardydiagnostics
USP 800 covers humidity control and hazard monitoring for hazardous drugs, personal protective equipment, medical monitoring, and other related links.
Table 8: USP 797
| No. | Humidity Range | Temperature Range | Equipment | Requirements |
| 1 | Relative humidity less than 60%; | Temperature range of 15° to 25° C; | l Aseptic preparation of hazardous and non-hazardous drugs;
l Includes injectable drugs and drugs for use in the patient's eye; |
l Requires monitoring of temperature and humidity;
l Requires monitoring of air pressure and air exchange rate; |
Table 9: USP 800
| No. | Humidity Range | Temperature Range | Equipment | Requirements |
| 1 | Relative humidity less than 60%; | Temperature range of 15° to 25° C; | l Aseptic and non-aseptic preparation of hazardous drugs;
l Aggressive drugs commonly used to treat cancer; |
l Requires monitoring of temperature, humidity, pressure, and air exchange rate; |
10.Why Humidity Is A Challenge For Pharmaceutical Industry?
To control the humidity for pharmaceutical industry is not difficult, but accurate environmental humidity control is vital. The humidity level in the pharmaceutical production process and production facilities affects the results of each stage of the process. The challenges are:
Humidity Challenges For Pharmaceutical Industry-sourced: searchenginejournal
Instability of Drug Ingredients
From research and development to manufacturing, packaging and storage, humidity control must be accurate at each stage, otherwise the unstable ingredients in the product will absorb moisture from the air, thereby reducing efficacy and even causing danger in some cases. Among them, antibiotics are a typical example.
Huge Economic Losses
Uncontrolled humidity will have various effects on the long-term stability and effectiveness of drugs, resulting in huge economic losses. Ultimately, it will affect the development and launch of new products.
Difficulty in Drug Storage
In addition to humidity control in research and development and production, humidity control after the drug is packaged is also crucial, including the transportation process and storage process is a huge test.
Endangering Public Safety
Drugs that are not accurately humidity controlled may breed bacteria and viruses, making the product toxic. This will pose a great threat to public safety.
Conclusion:
Humidity control is important in all areas of processing, especially in the pharmaceutical industry. In order to be able to achieve the highest product quality, you need to determine and control the humidity control of the environment at all times. If there are more you want to know about, please contact us now!
Don't forget to share this post!
Blister Packaging Machine Related Posts
Blister Packaging Machine Related Products
Blister Packaging Machine Related Videos
CONTACT US
Tell us your raw material and project budget to get quotations within 24 hours.
WhatsApp Us: +86 181 6426 8586

Want the best price & newest pharmaceutical machinery buying guide,tips and trends sent straightly to your box?Sign up for Aipak’s monthly newsletter,we’re free for your consultation and Offer you the most suitable solutions!
The Buyer's Guide
- Capsule Filling Buyer's Guide
- Blister Packaging Buyer's Guide
- Tablet Counting Buyer's Guide
- Tube Filling Buyer's Guide
- Cartoning Buyer's Guide
- Gummy Making Buyer's Guide
- CO2 Extraction Buyer's Guide
- Empty Capsules Buyer's Guide
- Suppository Filling Buyer's Guide
- Tablet Coating Buyer's Guide
- Tablet Press Buyer's Guide
- Softgel Encapsulation Buyer's Guide
Most Popular
- 7 Importance Of Pharmaceutical Packaging In Different Applications You Must Know
- 6 Advantages You Must Know About Tablet Counting Machine
- 8 Advantages of Blister Packaging You Must Know
- 6 Critical Applications of Automatic Capsule Filling Machine
- 6 Stations You must Know to Improve the Filling Quality of Automatic Capsule Filling Machine