Influenza Vaccine Actually Reduces COVID-19 Critical Conditions By 90%
COVID-19 and influenza are both infectious respiratory diseases with some similar symptoms, but they are caused by different viruses and there are some differences in the characteristics of people who become seriously ill and in the way they are treated.
Vaccination is an effective way to prevent infection, and neocoronaviruses and influenza viruses are not the same and have their own modes of action in terms of vaccine efficacy.
Recent studies have shown that influenza vaccination reduces severe neocon disease by 90%. It is important that we understand the differences between the New Coronavirus and the influenza virus in order to better protect ourselves.
Figure 1
On 16 May 2022, Nature published an article stating that the influenza vaccine reduces the risk of neo-crown infection, particularly in terms of protection against neo-crown reassortment, with 90% efficacy (Figure 1) .
If we knew in advance that a vaccine for influenza or other diseases could provide protection against COVID-19, then even if it was only a short time in advance, it could reduce deaths and injuries from future COVID-19 pandemics, saving millions of lives and allowing time for the development of a neo-crown vaccine.
Figure 2
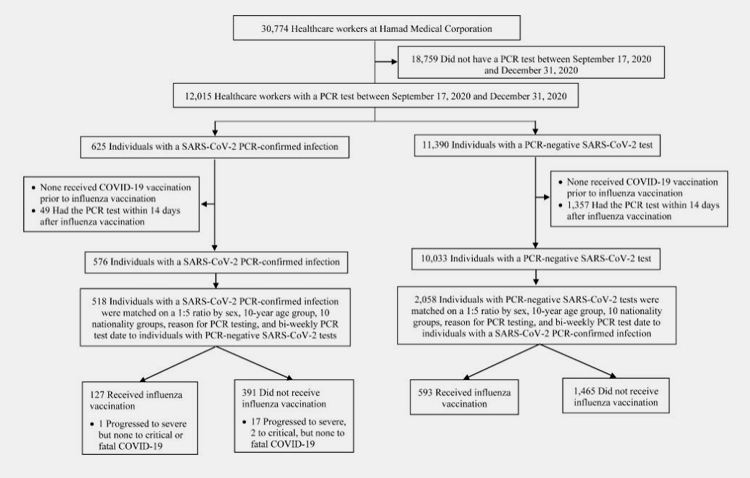
On 9 May 2022, a team of researchers from Weill Cornell Medical Center in Doha published a study entitled "Effectiveness of influenza vaccination against SARS-CoV-2 infection among healthcare workers in Qatar" (Figure 2) .
The study found that those who received the influenza vaccination were nearly 90% less likely to develop a new severe crown in the following months compared to those who had not recently received the influenza vaccination.
The study was led by infectious disease epidemiologist Laith Jamal Abu-Raddad and analysed the health records of 30,774 health workers in Qatar between 17 September 2020 and 31 December 2020 (the period before the COVID-19 vaccination and after the influenza vaccination).
Figure 3
The median age of the data sample was 36 years, the median age of the control group was 35 years, and the median duration between influenza vaccination and PCR (Polymerase Chain Reaction) testing was 43 days (Figure 3)
Analysis of the data results led to the following conclusions.
- The effectiveness of protection against the new coronavirus 14 days after influenza vaccination was 29.7%.
- The effectiveness of the influenza vaccine against any severe, critical or fatal symptoms of neocon was 88.9%
- Influenza vaccination was associated with a significant reduction in the risk of SARS-CoV-2 infection and in the severity of COVID-19
- The duration of protective efficacy of influenza vaccine against new coronas was approximately six weeks.
Günther Fink, an epidemiologist at the University of Basel, Switzerland, said: "Our previous findings in Brazil showed that influenza vaccination was associated with a reduced risk of death in patients hospitalised with COVID-19. This analysis from Qatar reduces the possibility that other studies that have found the same link are just flukes."
Mihai Netea, an infectious disease specialist at Radboud University Medical Centre in Nijmegen, the Netherlands, said, "This is important evidence that the flu vaccine is associated not only with a reduction in SARS-CoV-2 infection but also with disease severity, an observation that strongly suggests that this protection is real.
However, I don't think the protective effect of the influenza vaccine against new crowns lasts very long, perhaps as little as six months to two years. It is not entirely clear why an influenza vaccine consisting of inactivated influenza viruses also protects against neocon pneumonia. Vaccines train the immune system to recognise specific pathogens, but they also enhance broadly acting antiviral defences, and Laith Jamal Abu-Raddad has found signs of this response in influenza vaccine recipients."
What are the similarities and differences between influenza viruses and COVID-19?
A virus is a non-cellular organism that parasitises within living cells and proliferates in a replicative manner. Different viruses invade cells in different ways, but most require cellular internalisation through binding to specific receptor proteins or lipid structures on the cell surface to initiate the invasion process and infect the host cell.
Therefore, unravelling the specific processes and mechanisms by which viruses bind and internalise invading cells can help to develop targeted drugs or vaccines at the source.
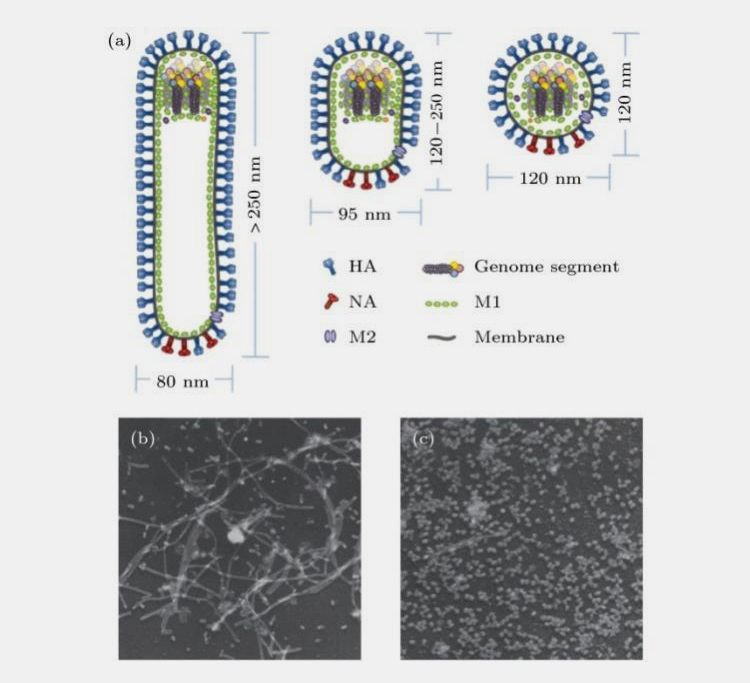
Influenza viruses can be classified into four types, A (A), B (B), C (C) and D (D), according to the antigenicity of their nucleoproteins. Human influenza is mainly caused by influenza A and B viruses, while influenza C viruses cause only insignificant or mild upper respiratory tract infections in humans and influenza D viruses are mainly hosted by cattle. Influenza viruses belong to the family Orthomyxoviridae and are enveloped viruses with three types of membrane proteins: Hemagglutinin (HA), Neuraminidase (NA) and Membrane protein 2 (M2), which exists as a homotrimer.
Figure 4
NA is a mushroom-shaped tetrameric glycoprotein with sialic acid-hydrolyzing activity that facilitates the release of virus from the host cell. Membrane protein M2 functions as an ion channel and regulates intra-membrane pH.
In addition, matrix protein M1 forms the outer shell skeleton of the virus and is tightly bound to the outermost envelope of the virus, serving to protect the core and maintain the spatial structure of the virus. The genetic material of the virus is a single stranded negative-stranded RNA folded together with nucleoproteins to form the viral ribonucleoprotein complex (Figure 4).
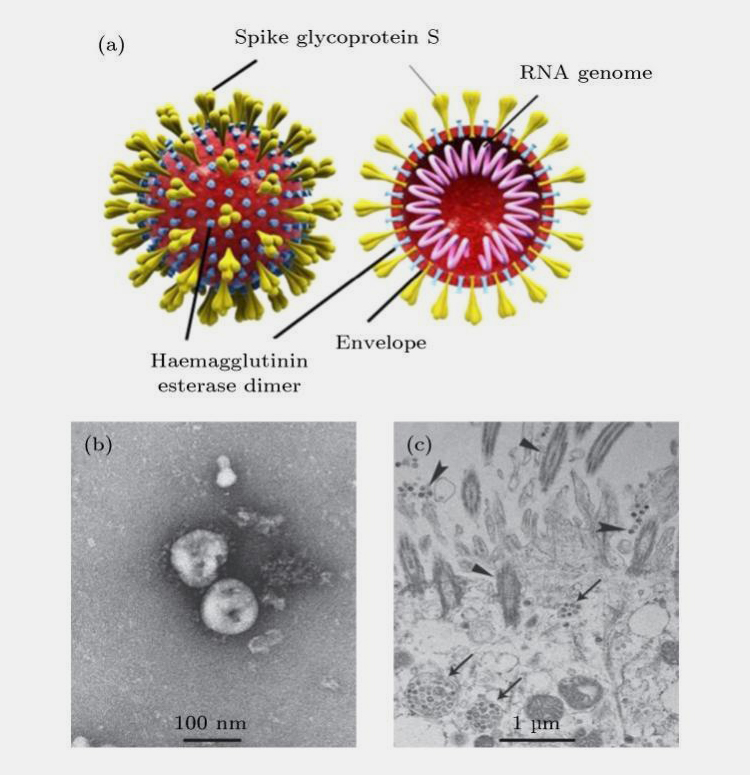
COVID-19 are a large group of viruses that are widespread in nature, infecting only vertebrates, and were first isolated from chickens. COVID-19 particles are approximately 60-220 nm in diameter and have three types of glycoprotein on their surface: spinosomal glycoprotein, small envelope glycoprotein, membrane glycoprotein and, to a lesser extent, haemagglutinin glycoprotein (Figure 5).
Figure 5
The nucleic acid of COVID-19 is linear single-stranded positive RNA with a methylated capsid structure at the 5' end and a polyA tail at the 3' end, similar to eukaryotic mRNA, which can function as a translation template on its own. With a full genome length of 27-32 kb, it is the largest genome of any known RNA virus.
The International Committee for the Classification of Viruses classifies them into four genera, namely alpha, beta, gamma and the newly postulated genus delta coronavirus. COVID-19 is a beta genus coronavirus with a high infectious capacity. The full genome sequence of the new coronavirus and the proteins that the virus binds to cells have been detected, but the mechanism by which it invades and infects cells is not known.
Influenza virus and SARS-CoV-2 both bind to the cell surface by ligand receptor binding and are activated by intracellular proteolytic enzymes to form two subunits of HA and S proteins, which are responsible for virus-host cell binding and mediating the membrane fusion process, respectively.
Influenza viruses require a variety of cytokines to bind to cellular receptors in order to endocytose, and it has been suggested that neocoronaviruses may also use intracellular pro-adsorption factors, such as binding to cellular glycoproteins, to enhance their infectivity.
There are some similarities in the mechanism of action of influenza viruses and COVID-19 on cells, so the use of influenza virus research methods to investigate the action of neocoronaviruses on cells is a potential avenue, and protection against COVID-19 by influenza vaccines is not difficult to understand.
Don't forget to share this post!
Bin Mixer Related Posts
Bin Mixer Related Products
Bin Mixer Related Videos
CONTACT US
Tell us your raw material and project budget to get quotations within 24 hours.
WhatsApp Us: +86 181 6426 8586
Want the best price & newest pharmaceutical machinery buying guide,tips and trends sent straightly to your box?Sign up for Aipak’s monthly newsletter,we’re free for your consultation and Offer you the most suitable solutions!
The Buyer's Guide
- Capsule Filling Buyer's Guide
- Blister Packaging Buyer's Guide
- Tablet Counting Buyer's Guide
- Tube Filling Buyer's Guide
- Cartoning Buyer's Guide
- Gummy Making Buyer's Guide
- CO2 Extraction Buyer's Guide
- Empty Capsules Buyer's Guide
- Suppository Filling Buyer's Guide
- Tablet Coating Buyer's Guide
- Tablet Press Buyer's Guide
- Softgel Encapsulation Buyer's Guide
Most Popular
- 7 Importance Of Pharmaceutical Packaging In Different Applications You Must Know
- 6 Advantages You Must Know About Tablet Counting Machine
- 8 Advantages of Blister Packaging You Must Know
- 6 Critical Applications of Automatic Capsule Filling Machine
- 6 Stations You must Know to Improve the Filling Quality of Automatic Capsule Filling Machine